Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6601
Revised: April 26, 2005
Accepted: April 30, 2005
Published online: November 14, 2005
AIM: To investigate the relationship between the expression levels of nm23 mRNA, CD44s, and CD44v6, and oncogenesis, development and metastasis of human gastric adenocarcinoma, colorectal adenocarcinoma, intraductal carcinoma of breast, and lung cancer.
METHODS: Using tissue microarray by immu-histochemical (IHC) staining and in situ hybri-dization (ISH), we examined the expression levels of nm23 mRNA, CD44s, and CD44v6 in 62 specimens of human gastric adenocarcinoma and 62 specimens of colorectal adenocarcinoma; the expression of CD44s and CD44v6 in 120 specimens of intraductal carcinoma of breast and 20 specimens of normal breast tissue; the expression of nm23 mRNA in 72 specimens of human lung cancer and 23 specimens of normal tissue adjacent to cancer.
RESULTS: The expression of nm23 mRNA in the tissues of gastric and colorectal adenocarcinoma was not significantly different from that in the normal tissues adjacent to cancer (P>0.05), and was not associated with the invasion of tumor and the pathology grade of adenocarcinoma (P>0.05). However, the expression of nm23 mRNA was correlated negatively to the lymph node metastasis of gastric and colorectal adenocarcinoma (r = -0.49, P<0.01; r = -4.93, P<0.01). The expression of CD44s in the tissues of gastric and colorectal adenocarcinoma was significantly different from that in the normal tissues adjacent to cancer (P<0.05; P<0.01). CD44v6 was expressed in the tissues of gastric and colorectal adenocarcinoma only, the expression of CD44v6 was significantly associated with the lymph node metastasis, invasion and pathological grade of the tumor (r = 0.47, P<0.01; r = 5.04, P<0.01). CD44s and CD44v6 were expressed in intraductal carcinoma of breast, the expression of CD44s and CD44v6 was significantly associated with lymph node metastases and invasion (P<0.01). However, neither of them was expressed in the normal breast tissue. In addition, the expression of CD44v6 was closely related to the degree of cell differentiation of intraductal carcinoma of breast (χ2 = 5.68, P<0.05). The expressional level of nm23 mRNA was closely related to the degree of cell differentiation (P<0.05) and lymph node metastasis (P<0.01), but the expression of nm23 gene was not related to sex, age, and type of histological classification (P>0.05).
CONCLUSION: Patients with overexpression of CD44s and CD44v6 and low expression of nm23 mRNA have a higher lymph node metastatic rate and invasion. In addition, overexpression of CD44v6 is closely related to the degree of cell differentiation. Detection of the three genes is able to provide a reliable index to evaluate the invasion and metastasis of tumor cells.
- Citation: Liu YJ, Yan PS, Li J, Jia JF. Expression and significance of CD44s, CD44v6, and nm23 mRNA in human cancer. World J Gastroenterol 2005; 11(42): 6601-6606
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6601.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6601
Tissue microarray (TMA) technology is a new method used to analyze hundreds of tumor samples on a single slide, allowing high resolution analysis of genes and proteins on a large cohort. TMA is ideally suitable for genomics-based diagnosis and drug target locating. Oncogenesis, development and metastasis of tumor are triggered by many genes and factors. In recent years, research on the molecular mechanism of oncogenesis and metastasis and the diagnosis of the correlated mark of tumor are important objects in clinical research in oncology. CD44 is one of the transmembrane proteins on the surface of cells. Its distribution is very extensive and can be detected in lymphocytes and fibroblasts[1]. The expression of CD44v, which is related to the progression, metastasis and prognosis of tumor, has been detected in lung cancer, carcinoma of colon, esophageal cancer, carcinoma of breast, carcinoma of urinary bladder, liver cancer, cervix cancer, carcinoma of kidney and reticulosarcoma[2-5]. Nm23 was first found by Steeg in 1988. Lower expression of nm23 is related to the metastasis of tumors, such as carcinoma of breast, lung cancer, gastric carcinoma, malignant melanoma, and ovary cancer, and has been regarded as the gene of transfer inhibition. In order to investigate the relationship between the expression level of nm23 mRNA, CD44s and CD44v6 and the oncogenesis, pathological grade, invasion, and metastasis of tumor, we detected the expression of nm23 mRNA, CD44s, and CD44v6 in tissues of human gastric adenocarcinoma, colorectal adenocarcinoma, intraductal carcinoma of breast and lung cancer, and also in tissues adjacent to cancer using tissue microarray by immunohistochemical (IHC) staining and in situ hybridization (ISH).
The following tissue specimens were enrolled in this study: 40 specimens of colorectal adenocarcinoma (including 16 specimens of moderately differentiated colorectal adenocarcinoma, 24 poorly differentiated adenocarcinoma, 14 lymph node metastatic carcinoma, and 17 invasive carcinoma) and 22 specimens of normal colorectal tissue, 40 specimens of gastric adenocarcinoma (including 20 specimens of moderately differentiated gastric adenocarcinoma, 20 poorly differentiated adenocarcinoma, 15 lymph node metastatic carcinoma, and 23 invasive carcinoma) and 22 specimens of normal gastric tissue, 120 specimens of intraductal carcinoma of breast (including 86 specimens of moderately differentiated intraductal carcinoma, 34 poorly differentiated intraductal carcinoma, 30 lymph node metastatic carcinoma and 58 invasive carcinoma) and 20 specimens of normal breast tissue, 72 specimens of lung cancer (including 13 specimens of moderately differentiated carcinoma, 19 poorly differentiated carcinoma, 24 lymph node metastatic carcinoma) and 23 specimens of normal lung tissue. All patients underwent surgery at Xijing Hospital of the Fourth Military University, China in 2003. The resected specimens were fixed in 10% formaldehyde.
CD44s and CD44v6 monoclonal antibodies were obtained from MBI. SABC kit was bought from Sina-America Biotechnology Company. nm23 oligonucleotide probe and CSA test kit were purchased from Boshide Biotechnology Company. Biotin labeled anti-digitoxin (DIG) antibody was obtained from Sigma.
Samples were fixed in buffered formaldehyde and subsequently paraffin-embedded. Histological sections (5 μm) were prepared from the specimens, and then diagnosed and labeled by the pathologist. Tissue microarray was performed on the instrument of microarray from Beecher, USA. A hole (diameter 0.5 mm) was made on the recipient block (blank paraffin block), then the tissue core was taken on the supply block (labeled tissue block) and put in the hole. The former procedure was repeated and tissue microarray was completed. Histological sections (3-5 μm) were prepared with the instrument of Leica, Germany, and then rediagnosed by the pathologist.
The sections for tissue microarray were dewaxed in water by normal technique, and then the antigen was restored in high pressure (at 121 °C for 10 min). Immunohistochemistry was performed with the SABC kit. The primary antibodies were CD44s and CD44v6 monoclonal antibodies. Staining was performed following the instructions of SABC kit.
All reagents and containers were treated by diethy-lprocarbonate (DEPC). In brief, the sections for tissue microarray were dewaxed in water by normal technique, immersed into 3% H2O2 at room temperature for 10 min, and then washed twice with distilled water. Twenty microgram per milliliter of freshly diluted protease K was added and digested for 20 min at 37 °C to expose mRNA nucleic acid segments. Twenty milliliter of glycerine (20%) was added to the dry bottom of the test kits to keep humidity, 20 μL of the pre-hybridization solution was added to each section and kept at 37 °C for 4 h. Then the supernumerary liquid was absorbed without washing, hybridization solution was added as mentioned above. The sections were then covered with protective membrane and put in homeothermia at 40 °C overnight. After the coverglass was removed, the sections were washed twice at 37 °C in 2×SSC for 5 min, once in 0.5×SSC for 15 min, once in 0.2×SSC for 15 min, and kept in 3% BSA at 37 °C for 30 min and in seal solution at 37 °C for 30 min. After being washed with 0.5 mol/L PBS, the biotin labeled anti-digitoxin antibody was added for 60 min at 37 °C, followed by biotin-peroxidase for 20 min at 37 °C and then washed with PBS. Color was showed by DAB. Finally, the sections were restained, dehydrated, pellucidated, and sealed. Negative controls were designed.
Specimens were considered positive when >50% of the tissue components were immunohistochemically stained brown-yellow in appropriate cellular compartment. Specimens were considered positive for ISH when >50% of the tissue components were stained blue in appropriate cellular compartment.
Statistical analysis was performed with χ2 test. P<0.05 was considered statistically significant.
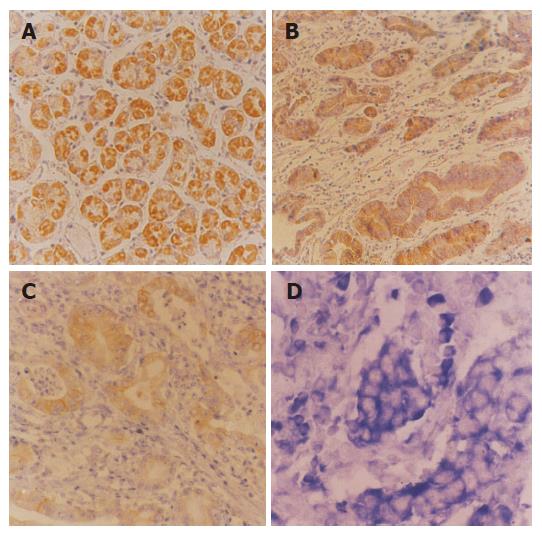
The expression of CD44s in tissues of colorectal adeno-carcinoma and normal colorectal mucosa was 42.0% (17/40) and 13.6% (3/22), respectively (Figures 1A and B), and there was a significant difference between them (χ2 = 5.18, P<0.05). In addition, there was a statistical significance between colorectal adenocarcinoma of with and without invasion (χ2 = 10.52, P<0.01), as well as with and without lymph node metastasis (χ2 = 12.48, P<0.01). However, there was no statistical significance between moderately and poorly differentiated colorectal adenocarcinoma (P>0.05), indicating that the expression of CD44s was related to the oncogenesis and development of colorectal adenocarcinoma. The expression of CD44v6 in tissues of colorectal adenocarcinoma and normal colorectal mucosa was 55.8% (22/40) and 0% (0/22), respectively (Figure 1C). There was a statistical significance between moderately and poorly differentiated colorectal adenocarcinoma (P<0.05) with and without invasion (P<0.05), as well as with and without lymph node metastasis (χ2 = 9.22, P<0.01). The results showed that the expression of CD44v6 was related not only to the invasion of colorectal carcinoma, but also to the metastasis. The expression of nm23 mRNA in tissues of colorectal adenocarcinoma and normal colorectal mucosa was 45.0% (18/40) and 40.9% (9/22), respectively (Figure 1D), and there was no statistically significant difference (P>0.05). However, the expression of nm23 mRNA in gastric adenocarcinoma with/without lymph node metastasis was 28.5% (4/14) and 53.8% (14/26), respectively. The difference was statistically significant (χ2 = 8.47, P<0.05). In addition, the expression of CD44v6 in colorectal adenocarcinoma was related to the invasion (P<0.05), but not to the pathological grade (P>0.05, Table 1).
| Pathologic | n | CD44s | CD44v6 | nm23 mRNA | |||
| feature | Positive (%) | P | Positive (%) | P | Positive (%) | P | |
| Grade | >0.05 | <0.05a | >0.05 | ||||
| II | 16 | 7(43.7) | 11(68.7) | 8(50.0) | |||
| I | 24 | 10(41.6) | 11(45.8) | 10(41.6) | |||
| Invasion | <0.01b | <0.01b | <0.05C | ||||
| + | 17 | 11(64.7) | 12(70.5) | 5(29.4) | |||
| – | 23 | 6(26) | 10(43.4) | 13(56.5) | |||
| Metastasis | <0.0d | <0.05e | <0.05e | ||||
| + | 14 | 9(64.2) | 10(71.4) | 4(28.5) | |||
| – | 26 | 8(30.7) | 12(46.1) | 4(53.8) | |||
| Age/yr | >0.05 | >0.05 | >0.05 | ||||
| >60 | 19 | 8(42.1) | 10(52.6) | 8(42.1) | |||
| <60 | 21 | 9(42.8) | 12(57.1) | 10(47.6) | |||
| Sex | >0.05 | >0.05 | >0.05 | ||||
| M | 26 | 10(38.4) | 14(53.8) | 12(46.1) | |||
| F | 7(50) | 8(57.1) | 6(42.8) | ||||
| Normal | 4(18.1) | 0(0) | 9(40.9) | ||||
Expression of CD44s and CD44v6 was associated with invasion and lymph node metastasis of colorectal adenocarcinoma (r = 0.47, P<0.05). However, expression of nm23 mRNA was not associated with invasion and lymph node metastasis of colorectal adenocarcinoma (r = -0.49, P<0.05), suggesting that CD44s, CD44v6 and nm23 mRNA could regulate invasion and lymph node metastasis of colorectal adenocarcinoma. In addition, the expression level of nm23 mRNA, CD44s and CD44v6 was not associated with the age and sex of patients (P>0.05, Table 1).
The expression of CD44s in tissues of gastric adeno-carcinoma and normal gastric mucosa was 48% (19/40) and 13.6% (3/22), respectively (Figures 2A and B), and there was a significant difference between them (χ2 = 10.29, P<0.01). However, the expression of CD44s was not significantly associated with lymph node metastasis. There was no statistical significance between moderately and poorly differentiated gastric adenocarcinoma with/without invasion (P>0.05). The expression of CD44v6 in tissues of gastric adeno-carcinoma and normal gastric mucosa was 63.3% (25/40) and 0% (0/22), respectively (Figure 2C). There was a statistical significance between moderately and poorly differentiated gastric adenocarcinoma (χ2 = 9.19, P<0.01) with and without invasion (χ2 = 22.22, P<0.01), as well as with and without lymph node metastasis (χ2 = 10.36, P<0.01). The expression of nm23 mRNA in tissues of gastric adenocarcinoma and normal gastric mucosa was 47% (19/40) and 43% (9/22), respectively (Figure 2D) and there was no statistically significant difference between them (P>0.05). However, the expression of nm23 mRNA in gastric adenocarcinoma with/without lymph node metastasis was 26.7% (4/15) and 60% (15/25) respectively, the difference was significant (χ2 = 18.47, P<0.01, Table 2).
| Pathologic | n | CD44s | CD44v6 | nm23 mRNA | ||||||
| feature | Positive | % | P | Positive | % | P | Positive | % | P | |
| Grade | >0.05 | b<0.01 | >0.05 | |||||||
| II | 20 | 8 | 40.0 | 10 | 50.0 | 9 | 45.0 | |||
| III | 20 | 10 | 50.0 | 15 | 75.0 | 10 | 50.0 | |||
| Invasion | >0.05 | d<0.01 | >0.05 | |||||||
| + | 23 | 11 | 47.8 | 18 | 78.2 | 11 | 47.8 | |||
| – | 17 | 7 | 41.1 | 7 | 41.0 | 8 | 47.1 | |||
| Metastasis | >0.05 | f<0.01 | f<0.01 | |||||||
| + | 15 | 6 | 40.0 | 12 | 80.0 | 4 | 26.7 | |||
| – | 25 | 12 | 48.0 | 13 | 52.0 | 15 | 60.0 | |||
| Age/year | >0.05 | >0.05 | >0.05 | |||||||
| >60 | 21 | 10 | 47.2 | 13 | 61.9 | 9 | 42.9 | |||
| <60 | 19 | 8 | 42.1 | 12 | 63.1 | 10 | 52.6 | |||
| Normal | 22 | 3 | 13.6 | 0 | 0 | 9 | 43.0 | |||
The expression of CD44v6 was associated with lymph node metastasis of gastric adenocarcinoma (r = 5.04). However, the expression of nm23 mRNA was not associated with lymph node metastasis of gastric adenocarcinoma (r = -4.93, Table 2).
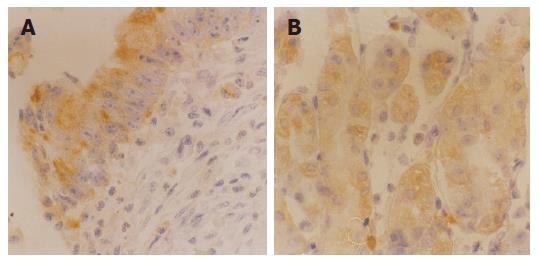
The expression of CD44s and CD44v6 in tissues of intraductal carcinoma of breast was 45.8% (55/120) and 53.3% (64/120), respectively (Figures 3A and B), but neither of them was expressed in normal breast tissue. The expression of CD44s and CD44v6 in intraductal breast carcinoma with and without invasion had a significant statistical difference (χ2 = 9.52, P<0.01; χ2 = 22.89, P<0.001), as well as with and without lymph node metastasis (χ2 = 9.41, P<0.01; χ2 = 8.75, P<0.01). Furthermore, the expression of CD44s was not significantly associated with moderately and poorly differentiated intraductal breast carcinoma (P>0.05). However, the expression of CD44v6 was not only related to invasion and lymph node metastasis of intraductal breast carcinoma, but also related to its pathological grade (χ2 = 5.68, P<0.05, Table 3).
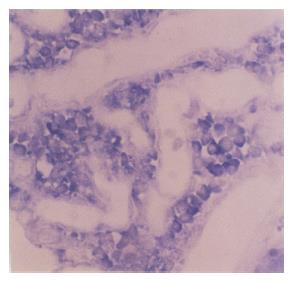
The expression of nm23 mRNA in tissues of lung cancer and normal lung tissues was 55.2% (40/72) and 82.6% (19/23), respectively (Figure 4), and there was a significant difference between them (χ2 = 5.42, P<0.05). The expression of nm23 mRNA in tissues of lung cancer with and without lymph node metastasis was 25.0% (6/24) and 70.8% (34/48), respectively and there was a significant difference between them (χ2 = 13.61, P<0.001), as well as between moderately and poorly differentiated lung cancer (χ2 = 9.61, P<0.01), indicating that the expression of nm23 mRNA was associated with the oncogenesis, lymph node metastasis and pathological grade of lung cancer. In addition, the expression of nm23 mRNA in lung cancer was not associated with the age and sex of patients and the pathologic feature of lung cancer (P>0.05, Table 4).
CD44s, a hyaluronic acid receptor, is important in regulating invasion and metastasis of tumor[3-7]. Previous studies demonstrated that the expression of CD44s is an important biological marker for predicting metastatic potential. Invasion and metastasis of tumor are a very complicated process, which is regulated by many correlated genes. CD44v6 may take part in the invasion and meta-stasis of tumor, but it is not the necessary factor for tumor invasion and metastasis. When CD44v6 produces a marked effect, it must be restricted by many factors in vivo. It has been proved that CD44v6 is correlated to tumor invasion and metastasis in experiments of cultured cells and animals. But consistent view has not been reached so far with respect to the relationship between expression of CD44v6 and human tumor invasion and metastasis.
It was reported that CD44v mRNA is expressed in human colorectal carcinoma as detected by RT-PCR[8-10]. Zalewski et al[11] reported that the expression of CD44 is associated with the size of tumor, age, and sex of patients. In our current study, CD44 and CD44v6 were expressed in colorectal carcinoma and normal colorectal tissues respectively, indicating that the expression of CD44 and CD44v6 is associated with the invasion and metastasis of colorectal carcinoma, but not associated with the age and sex of colorectal carcinoma patients. In addition, the expression of CD44v6 was related to the pathological grade of tumor. Other studies reported that the expression of CD44 is not associated with the clinical pathological feature of colorectal carcinoma[12,13]. Whether the expression level of CD44 can be seen as a marker of tumor histopathology requires further research.
Using reverse transcription polymerase chain reaction followed by Southern blotting, Yamamichi et al[14] exa-mined the expression of the standard and variant forms (v6 and v9) of CD44 mRNA in 73 cases of gastric cancer, and found that the expression status of the standard form of CD44 mRNA is correlated with peritoneal dissemination only, and that of CD44v9 mRNA does not significantly correlate with any clinicopathologic factor. Li et al[15] reported that 74% gastric cancers and 80% invasive carcinomas are positive for CD44v6, implying that CD44v6 is also a useful marker of tumor invasion and metastasis. In the present study, the expression of CD44v6 was significantly correlated with tumor differentiation, lymph node metastasis and invasion of tumor. The expression of CD44s and CD44v6 in gastric cancer was much higher than that in normal stomach and was associated with genesis, metastasis and clinically aggressive behavior of gastric adenocarcinoma.
The aberrant activation of two or more genes plays a different role during different stages of genesis, pro-gression and metastasis of malignant tumor. These genes synergically facilitate carcinoma change. Our studies showed that the expression of nm23 mRNA in colorectal adenocarcinoma was negatively correlated with that of CD44 and CD44v6, which was closely associated with the invasion and metastasis of colorectal adenocarcinoma. These results indicate that nm23 and CD44v6 synergically play positive and negative roles during lymph node metastasis of colorectal, and gastric adenocarcinoma. Further studies are needed to investigate the relationship between nm23 and CD44v6, which is important for tumor metastasis.
Mammary cancer is one of the most common malignant tumors. Although 50% of mammary cancers are surgically curable, 50% patients have metastasis within 5 years after surgery. Kaufmann et al[16] reported that the positive expression rate of CD44v6 is 80% in primary tumor and 100% in focal tumor, but no expression is found in normal mammary tissue. The patients with positive expression of CD44v6 have a poor prognosis. There are still many correlative reports[17-20]. Our results showed that the expression of CD44s and CD44v6 in intraductal carcinoma was related with tumor invasion and metastasis, but not with the age and sex of patients.
Since the gene of nm23 was found, lower expression of nm23 has been found to be associated with high metastatic potential and poor prognosis of human mammary cancer, gastric cancer, lung cancer, melanoma, and ovary cancer. Nm23H1 is more important than nm23H2. Gazzeri et al[21] reported that the expression of nm23H1 is not associated with clinicopathological features of adenocarcinoma, while it is associated with the development of squamous carcinoma. Lai et al[22] reported that the expression of nm23H1 is positively correlated with tumor metastasis and prognosis. The lower the nm23H1 expression is, the poorer the prognosis is. Our studies showed that the expression of nm23 mRNA was correlated with lymph node metastasis (r = -4.93). The expression of nm23 mRNA was gradually reduced in normal lung tissue adjacent to cancer. In conclusion, nm23H1 may take part in the regulation of metastasis as a prohibitive gene, and may become a valuable target in the evaluation of tumor development and prognosis.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Gu H, Ni C, Zhan R. The expression of CD15 mRNA CD44v6 mRNA and nm23H1 mRNA in breast cancer and their clinical significance. Zhonghua YiXue ZaZhi. 2000;80:854-857. [PubMed] |
| 2. | Wang FL, Wei LX. Expression of CD44 variant exon 6 in lung cancers. Zhongguo YiXue KeXueYuan XueBao. 2001;23:401-402. [PubMed] |
| 3. | Yan RL, Qian XH, Xin XY, Jin M, Hui HX, Wang DT, Wang J. Experimental study of anti-VEGF hairpin ribozyme gene inhibiting expression of VEGF and proliferation of ovarian cancer cells. Ai Zheng. 2002;21:39-44. [PubMed] |
| 4. | Ylagan LR, Scholes J, Demopoulos R. Cd44: a marker of squamous differentiation in adenosquamous neoplasms. Arch Pathol Lab Med. 2000;124:212-215. [PubMed] |
| 5. | Shimabukuro K, Toyama-Sorimachi N, Ozaki Y, Goi T, Furukawa K, Miyasaka M, Aso T. The expression patterns of standard and variant CD44 molecules in normal uterine cervix and cervical cancer. Gynecol Oncol. 1997;64:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Frank S, Rihs HP, Stöcker W, Müller J, Dumont B, Baur X, Schackert HK, Schackert G. Combined detection of CD44 isoforms by exon-specific RT-PCR and immunohistochemistry in primary human brain tumors and brain metastases. Biochem Biophys Res Commun. 1996;222:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Montgomery E, Abraham SC, Fisher C, Deasel MR, Amr SS, Sheikh SS, House M, Lilliemoe K, Choti M, Brock M. CD44 loss in gastric stromal tumors as a prognostic marker. Am J Surg Pathol. 2004;28:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Kuniyasu H, Oue N, Tsutsumi M, Tahara E, Yasui W. Heparan sulfate enhances invasion by human colon carcinoma cell lines through expression of CD44 variant exon 3. Clin Cancer Res. 2001;7:4067-4072. [PubMed] |
| 9. | Yamada Y, Itano N, Narimatsu H, Kudo T, Hirohashi S, Ochiai A, Tohnai I, Ueda M, Kimata K. CD44 variant exon 6 expressions in colon cancer assessed by quantitative analysis using real time reverse transcriptase-polymerase chain reaction. Oncol Rep. 2003;10:1919-1924. [PubMed] |
| 10. | Masaki T, Goto A, Sugiyama M, Matsuoka H, Abe N, Sakamoto A, Atomi Y. Possible contribution of CD44 variant 6 and nuclear beta-catenin expression to the formation of budding tumor cells in patients with T1 colorectal carcinoma. Cancer. 2001;92:2539-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Zalewski B, Famulski W, Sulkowska M, Sobaniec-Lotowska M, Piotrowski Z, Kisielewski W, Sulkowski S. CD44 expression in colorectal cancer. An immunohistochemical study including correlation with cathepsin D immunoreactivity and some tumour clinicopathological features. Folia Histochem Cytobiol. 2001;39 Suppl 2:152-153. [PubMed] |
| 12. | Sökmen S, Lebe B, Sarioglu S, Füzün M, Terzi C, Küpelioglu A, Ellidokuz H. Prognostic value of CD44 expression in colorectal carcinomas. Anticancer Res. 2001;21:4121-4126. [PubMed] |
| 13. | Ishida T. Immunohistochemical expression of the CD44 variant 6 in colorectal adenocarcinoma. Surg Today. 2000;30:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Yamamichi K, Uehara Y, Kitamura N, Nakane Y, Hioki K. Increased expression of CD44v6 mRNA significantly correlates with distant metastasis and poor prognosis in gastric cancer. Int J Cancer. 1998;79:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Li H, Li J, Guo L. Characteristics of expression of CD44v and receptor for HA-mediated motility (RHAMM) in multi-step gastrocarcinogenesis. Zhonghua ZhongLiu ZaZhi. 1999;21:329-331. [PubMed] |
| 16. | Kaufmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995;345:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 281] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Kopp R, Classen S, Wolf H, Gholam P, Possinger K, Wilmanns W. Predictive relevance of soluble CD44v6 serum levels for the responsiveness to second line hormone- or chemotherapy in patients with metastatic breast cancer. Anticancer Res. 2001;21:2995-3000. [PubMed] |
| 18. | Saddik M, Lai R. CD44s as a surrogate marker for distinguishing intraductal papilloma from papillary carcinoma of the breast. J Clin Pathol. 1999;52:862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Sanchez Lockhart M, Hajos SE, Basilio FM, Mongini C, Alvarez E. Splice variant expression of CD44 in patients with breast and ovarian cancer. Oncol Rep. 2001;8:145-151. [PubMed] |
| 20. | Berner HS, Nesland JM. Expression of CD44 isoforms in infiltrating lobular carcinoma of the breast. Breast Cancer Res Treat. 2001;65:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Gazzeri S, Brambilla E, Negoescu A, Thoraval D, Veron M, Moro D, Brambilla C. Overexpression of nucleoside diphosphate/kinase A/nm23-H1 protein in human lung tumors: association with tumor progression in squamous carcinoma. Lab Invest. 1996;74:158-167. [PubMed] |
| 22. | Lai WW, Wu MH, Yan JJ, Chen FF. Immunohistochemical analysis of nm23-H1 in stage I non-small cell lung cancer: a useful marker in prediction of metastases. Ann Thorac Surg. 1996;62:1500-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |