Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6395
Revised: March 23, 2005
Accepted: March 24, 2005
Published online: October 28, 2005
AIM: To investigate the treatment efficacy of radiofrequency ablation (RFA) of hepatic malignant tumor and the relevant complications.
METHODS: A total of 338 patients with 763 hepatic tumors underwent ultrasound-guided RFA (565 procedures). There were 204 cases of hepatic cellular carcinoma (HCC) with 430 tumors, the mean largest diameter was 4.0 cm. Of them, 48 patients (23.5%) were in stages I-II (UICC Systems) and 156 (76.5%) in stages III-IV There were 134 cases of metastatic liver carcinoma (MLC), with 333 metastases in the liver, the mean diameter was 4.1 cm, the liver metastases of 96 patients (71.6%) came from gastrointestinal tract. Ninety-three percent of the 338 patients were treated using the relatively standard protocol. Crucial attention must be paid to monitor the abnormal changes in ultrasound images as well as the vital signs of the patients to find the possible hemorrhage and peripheral structures injury in time. The tumors were considered as ablated completely, if no viability was found on enhanced CT within 24 h or at 1 mo after RFA. These patients were followed up for 3-57 mo.
RESULTS: The ablation success rate was 93.3% (401/430 tumors) for HCC and was 96.7% (322/333 tumors) for MLC. The local recurrence rate for HCC and MLC was 7.9% (34/430 tumors) and 10.5% (35/333 tumors), respectively. A total of 137 patients (40.5%) underwent 2-11 times of repeated ablations because of tumor recurrence or metastasis. The 1st, 2nd, and 3rd year survival rate was 84.6%, 66.6%, and 63.1%, respectively; the survival rate from 48 patients of I-II stage HCC was 93.7%, 80.4%, and 80.4%, respectively. The major complication rate in this study was 2.5% (14 of 565 procedures), which consisted of 5 hemorrhages, 1 colon perforation, 5 injuries of adjacent structures, 2 bile leakages, and 1 skin burn.
CONCLUSION: RFA, as a minimally invasive local treatment, has become an effective and relatively safe alternative for the patients of hepatic malignant tumor, even of advanced liver tumor, tumor recurrence, and liver metastases. Knowledge about possible complications and their control may increase the treatment efficacy and help to promote the use of RFA technique.
- Citation: Chen MH, Yang W, Yan K, Gao W, Dai Y, Wang YB, Zhang XP, Yin SS. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol 2005; 11(40): 6395-6401
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6395.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6395
The treatment efficacy of radiofrequency ablation (RFA) of liver tumors has been confirmed by sufficient quantity of clinical results[1-4]. With the improvement in both equipments and treatment skills, RFA has gradually been developed in China to treat large tumors[5,6], and provided an efficient alternative for the tumors which were difficult to manage with conventional treatments. With the wide adoption of the RFA technique, the complications increased accordingly, which drew a very important clinical attention. Large tumor and advanced tumor were commonly seen in China. Taking into consideration the characteristics of the tumors of our contrary study, this research aimed to summarize the treatment efficacy of RFA in 338 malignant liver tumors and explore the effective measures for complications.
From 1999 to 2004, 338 patients with 763 hepatic tumors underwent 565 US-guided percutaneous RFA. All patients were diagnosed by biopsy at least on one lesion. Two hundred and forty-four patients were men (mean age, 59.1 years; age range, 24-87 years) and 94 were women (mean age, 58.6 years; age range, 32-86 years). Two hundred and four were hepatic cellular carcinoma (HCC) patients (430 tumors), with mean diameter of 4.0 cm (range, 1.2-10.8 cm). Of these 204 HCC patients, 96, 95, and 13 had Child-Pugh class A, B, and C cirrhosis, respectively. According to the UICC-TNM staging system, 48 patients (23.5%) were in stages I-II, 156 were in stages III-IV. In the 204 HCC patients, 29 were given palliative treatment because of large size, unclear tumor border, invasion to the surrounding anatomical structure or multi-tumor occurrence. One hundred and thirty-four patients (333 tumors) had metastatic liver carcinomas (MLCs) with mean diameter of 4.1 cm (range, 1.0-10.0 cm). The primary tumors were from gastric and colorectal tract (n = 96, 71.6%), breast cancer (n = 16), lung cancer (n = 10), pancreatic cancer (n = 5), other organs (n = 7), respectively. Nine of them were given palliative treatment because of large size or multi-tumors. Eleven patients were found to have extrahepatic metastases when the first RFA was performed. The tumors larger than 3.5 cm, accounted for 61.8% (209/338 patients) in this study. There were 51 patients (15.1%) with 54 tumors adjacent to the gastrointestinal tract and 46 patients (13.9%) with 51 tumors adjacent to the gallbladder in this group.
The RF system used in this study was a 460-kHz generator (Model 1500; RITA Medical System, Mountain View, CA, USA) that is capable of delivering a maximum power of 150 W through a 14-G electrode. The electrode contained nine hook-shaped prongs that could be deployed from the cannula, when the cannula was inserted into the surface of the tumor. The high-frequency electric current was passed through the deployed prongs to the tumor tissue, causing the vibration and friction of ions in the tissue, resulting in the temperatures of up to 100 °C, which produced a sphere-like coagulation area of 2.0-5.0 cm in diameter. The time to produce a 5-cm ablation sphere was about 20 min. If multiple-overlapping ablations were required to ablate large tumors, much more time was needed.
Ninety-three percent of the patients were treated using the established protocol. The ablation area should cover the tumor and at least 0.5-1.0 cm of the surrounding tissue, and the margin should even be more than 1 cm when the tumor border was unclear. The treatment protocol was decided according to the ablation range. The ablation number and relevant placement mode should be decided, if the ablation range was greater than 4.5 cm in diameter[6]. For example, a regular tetrahedron overlapping mode with 4 ablations should be used, if the ablation range was 5.1-5.3 cm; the regular three- to six-sided prism overlapping mode with 5-8 ablations should be adopted, if the ablation range was 5.4-6.6 cm; the three segment overlapping ablation mode with 12 ablations was needed, if the ablation range was 6.7-7.5 cm. Furthermore, the sequence of the ablations and the skill of electrode placement were also crucial[7].
During the ablation process, real-time ultrasound scan was performed to monitor the electrode placement and the ablation, and post-ablation ultrasound was performed to find the potential complications so that the corresponding measures could be carried out in time[8].
All patients underwent percutaneous RFA under general anesthesia or conscious sedation. Conscious sedation was induced with intravenous administration of 2.5-5.0 mg of midazolam (Roche; Basel, Switzerland) and 50-100µg of fentanyl (Fentaini; Renfu, Yichang, China). Local infiltration anesthesia was induced by 5-15 mL of 1% lidocaine (Liduokayin; Yimin, Beijing, China). When tumors were adjacent to the diaphragm or the liver surface, an intravenous bolus of propofol (Diprivan; Zeneca, Macclesfield, UK, 1-2 mg/kg) and fentanyl (50-100µg) was given to enhance anesthesia in combination with local anesthesia.
For irregular tumors larger than 5 cm and near the gastrointestinal tract, enhanced CT within 24 h after the treatment was used to detect any residual viable tissue that would require a second RFA and to observe possible complications. To evaluate the tumor response to RFA therapy, contrast-enhanced CT was performed 1 mo post-ablation and the complete ablation of the tumor was considered to be achieved if the scans revealed: (1) the ablation zone was beyond the tumor borders, (2) the margin of the ablation zone was clear and smooth, and (3) no contrast enhancement was detected within or around the tumor. Subsequently, the patients were monitored regularly for intrahepatic recurrence in the outpatient clinic by a follow-up protocol including serum α-fetoprotein (AFP), abdominal US and enhanced CT every 2-3 mo for the first year, and then AFP every 2-3 mo, abdominal US and enhanced CT every 4-6 mo after the first year. Suspected intrahepatic recurrence was confirmed by percutaneous fine needle biopsy. Local recurrence was defined as tumor recurrence within or at the periphery of the ablated lesion in the follow-up CT scan. Distant intrahepatic recurrence referred to a new tumor that appeared in the liver separated from the ablated area. Patients were followed-up for 3-57 mo, follow-up rate was 91.4% (309 patients).
Cumulative survival rate of each group was calculated using the Kaplan-Meier method and compared by log-rank test. The level of significance was set at 0.05 for all tests. SPSS statistical analysis software (SPSS, Chicago, IL, USA) was used.
The ablation success rate after the first RFA based on the CT findings were: HCC 93.3% (401/430 tumors), MLC 96.7% (322/333 tumors), the results are detailed in Table 1.
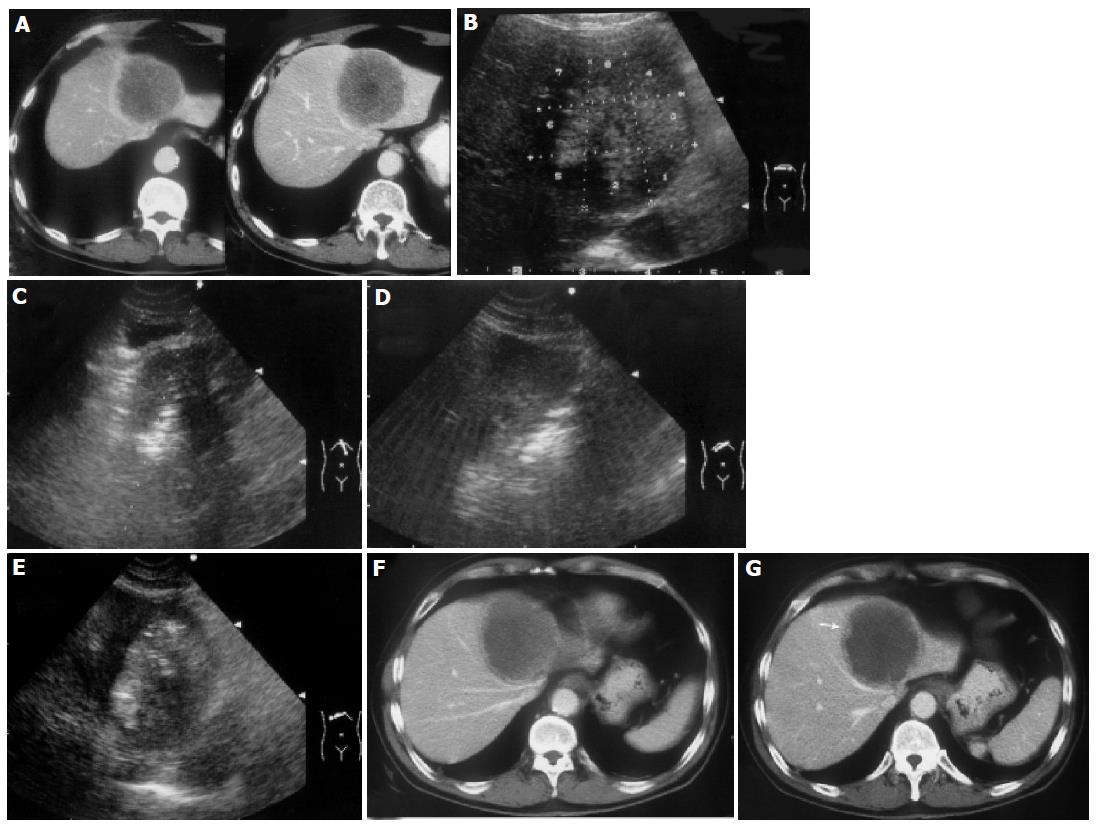
11.2% (38/338 cases) of the patients in this study had tumor residue, of which 31 cases (81.6%) were those with tumors larger than 3.5 cm; success rate for patients with tumors larger than 3.5 cm was 85.2% (178/209 cases, Figure 1). For tumor adjacent to bowel or gallbladder, the ablation success rates were 83.3% (45/54 tumors) and 86.3% (44/51 tumors), respectively. Local tumor recurrence was found in 34 of HCC patients (7.9%), 35 of MLC patients (10.5%), respectively, after the follow-up of 3-57 mo. 40.5% of patients received additional 2-11 RFA treatments because of the metastases or recurrence. The overall survival rate of 300 patients with malignant liver tumor after RFA is shown in Table 2.
The overall survival rate was higher in HCC group than in MLC group (P<0.001), so was the survival rate in HCC group of stages III-IV than in MLC group (P<0.05); and the survival rate in HCC group of stages I-II than in HCC group of stages III-IV (P<0.001).
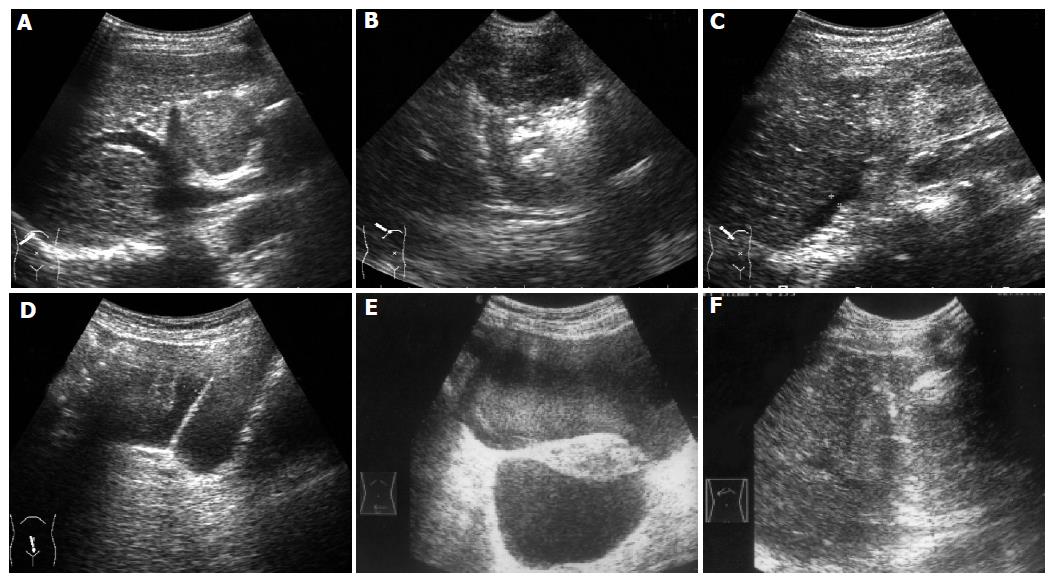
The occurrence rate of major complications was 2.5% (14/565 RFA procedures), including hemorrhage (n = 5, Figure 2), bile leakage (n = 2), colon perforation (n = 1), stricture of bile duct (n = 2), cholecystitis (n = 1), hemothorax (n = 2), skin burn (n = 1), minor complications consisted of bellyache, shoulder pain, breath-related right-superior bellyache, fever after RFA, transient hepatic dysfunction, etc.
All the therapeutic alternatives commonly used to treat HCC or MLC have limitations. In the recent decade, US-guided percutaneous ethanol injection (PEI) as a local therapy for hepatic tumors has gradually been replaced by intraoperative or percutaneous RFA[2,9,10], which was considered by many as superior to other existing locoregional therapies such as PEI, TACE, and microwave ablation[9,11,12]. However, the efficacy of RFA was reduced by its high recurrence rate and relevant complications, especially in the treatment of large tumors or special location tumors. Livraghi et al[13], reported complications of RFA in 2 320 patients with focal liver cancer treated by a collaborative group in Italy, and demonstrated that bleeding and gastrointestinal perforation were the most common major complications which might lead to death. Thus, studies on the treatment protocol of RFA to increase its efficacy and the preventive measures of the relevant complications have become a major issue.
Generally, a single ablation of 5 cm in diameter could successfully destroy a liver tumor smaller than 3.5 cm plus the “safety margin” of 0.5-1.0 cm. Patients with tumors larger than 3.5 cm accounted for 61.8% (207/335 cases) of the whole population in this study. With the help of the correct electrode placement, the operation skills and the established protocol, we got a response rate of 85.2% (178/209 cases), indicating that the protocol could be used to guide the RFA of large liver tumors[7]. However, this mathematic protocol was not always easy to perform in practice because of some factors, such as deviation of needle placement or failure of full deployment of prongs, due to rigid texture of the tumor or hepatic parenchyma, limitation of puncture angle, or insufficient safe margin due to being adjacent to major structures. For treating tumors with undesirable locations such as being adjacent to diaphragm, gallbladder, and gastrointestinal tract, etc. an individualized protocol was required. Some adjunctive measures or combination treatment of multiple modalities were also adopted to deal with particular features of the tumors[7,14].
Regarding the tumors being adjacent to diaphragm, gallbladder, gastrointestinal tract, the puncture direction of the electrode should be vertical to the organs and the cannular should be lifted a little, while the prongs had been deployed, besides multiple-overlapping ablations of 2-3 cm should be performed to ablate the risky area of tumor. If necessary, enhanced CT was performed within 24 h to detect residual tumor and decided if additional ablations were required. For the tumors adjacent to the colon, most part of the tumor which was near to the center of the liver should be ablated first, stage II resection should be adopted 2-4 wk later. Combination treatments including the TACE or obstruction of the liver arteries prior RFA should be adopted, if the tumor had abound blood supply; anyway, combination treatments were very important to improve the therapeutic efficacy[15].
There are many factors that can affect the therapeutic efficacy of RFA. The most important factor is the size of the tumor. Sufficient data confirmed that RFA could successfully ablate the small liver tumors. The ablation success rate of the initial RFA in this study was 94.8% (723/763 tumors). Of the 40 incompletely ablated tumors, 33 (82.5%) were larger than 3.5 cm. The ablation success rate of RFA on MLC patients reached 96.7% (322/333 tumors). It was considered that the MLC tumor was more sensitive to heat energy due to less blood supply[16]. Furthermore, in our study, most MLCs were treated with extended ablation margin as there were enough liver function reserve and surrounding liver tissue, thus contributing to a satisfactory short-term outcome of MLC in this study. In this study, six patients were found to have 1-3 MLC as well as primary stomach or colon cancer. The primary cancer had been successfully resected before RFA. Therefore, as an adjuvant therapy to the surgical resection, RFA could increase the opportunity of surgical resection of those patients with advanced liver tumor. Although additional RFA had been performed due to the new metastases in the liver after RFA, four of six patients had survived beyond 2-3 years. Although most of the HCC patients in our study were in stages III-IV, the 1-, 2- and 3- year survival rates after RFA was 84.6%, 66.6%, 63.1%, respectively, and 93.7%, 80.4%, 80.4% for HCC patients of stages I-II. These results indicated that RFA could have a similar efficacy compared with surgery resection as long as the tumors were completely ablated. However, the long-term efficacy of MLC post RFA was no good, the survival rate of 2nd, 3rd year was lower than that of HCC. Solbiati et al[3], reported that the average survival of MLC patients after RFA was 35.4 mo, which was higher than that of ours. It might be explained by the reason that most MLC patients in our study had large sized tumor, multi-tumor or extrahepatic metastases.
Another major factor to affect the efficacy was the undesirable location of the tumor, such as being adjacent to hepatic hilum, diaphragm, gastrointestinal tract or invasion to large vessels, or intruding outward from the liver surface, and RFA on those tumors may more likely induce tumor residue or complications.
More complications about RFA have been reported as more patients have been treated, despite that RFA is a minimally invasive technique[13,17]. The major complication rate in this study was 2.5% (14/565 procedures), which consisted of mechanical and thermal injuries. It is very important to find the auspice symptoms in time and adopt appropriate management.
Two of the five cases of hemorrhage occurred during the RFA treatment. One was due to liver capsule laceration when the electrode was deployed to treat a 1-cm metastatic liver tumor near the liver surface but unfortunately was pushed deep forward, and the anechoic area of hemorrhage in front of the liver enlarged to 1-3 cm in thickness in 2 min. The other case with metastatic tumor from rectum cancer was the result of stabbing subcapsular arterial-venous shunt by the RFA electrode and the anechoic area of hemorrhage increased to 2.5 cm in thickness. The above two cases of hemorrhage were stopped by using RFA at the bleeding sites without moving and the tumors were ablated successfully at the same time. The other three cases of hemorrhage were all HCC with liver cirrhosis. One hemorrhage occurred in a small HCC patient and was controlled by ablating the bleeding site. The other two occurred in patients with large liver tumors located near the liver surface and deteriorated coagulation functions. The hemorrhage was caused by the increased abdominal pressure due to cough or sudden position change at half or 4 h after the RFA treatment. The bleeding was controlled by hemostatics intravenously and local oppression and in one case whose bleeding amount was large that the patient even received blood transfusion of 800 mL.
Colon perforation occurred in one case in our early practice. The patient with HCC located in segment VI received two sessions of RFA procedure and a new 4-cm tumor near the colon hepatic flexure was found 1 year later. Contrast enhanced US demonstrated rich blood flow within the tumor, which was adhered to the colon. Overlooking this phenomenon, the tumor was ablated aggressively. Colon perforation occurred 1 wk later and the patient underwent surgical operation. In retrospect, we reckoned that the perforation might have been avoided if an individualized treatment protocol had been adopted with greater care as follows: (A) the patients were placed in the right anterior oblique position, so that the transverse colon and intestines would migrate to the left, away from the liver. (B) During the ablation, the patients were instructed to make repeated abdominal breaths to keep the bowels moving and to avoid continual hyperthermia in the bowel wall. (C) After the ablation, the patient needs to fast for 24 h and then has semi-fluid diets for 2 d. If the patient complains about right upper abdominal pain or has thickened bowel wall demonstrated by US or CT examination 24-48 h after the treatment, ask the patient to fast longer and receive infusion and other conservative treatment. In our later series of 51 patients with liver tumors near the bowels and 3 mo later, 8 (15.7%) patients demonstrated bowel adhesion but no colon perforation occurred.
Other adjacent structure injury occurred in eight cases. One patient with gallbladder stone developed cholecystitis and recovered after antibiotic administration. For tumors near the gallbladder, RFA might lead to a series of syndromes caused by parasympathetic stimulation with decreased heart rate and pain in stomach or pericardial area. Of the 46 cases with tumors near the gallbladder, 38 (82.6%) complained of pain in stomach and pericardial area, 17 (36.9%) had decreased heart rate with the lowest being 27 per min. Enhancing anesthesia and injecting of atropine of 0.25-1.0 mg could control these disorders. Two patients with slightly dilated bile duct due to tumor invasion had jaundice and obviously dilated bile duct after the RFA. Both recovered after percutaneous transhepatic biliary drainage.
Diaphragm injury occurred in two cases. One with bloody pleural infusion was given hemostatics and drainage of pleural cavity. The other with large amount of pleural effusion and thickened diaphragm recovered after draining out pink effusion fluid.
Two cases of biliary leakage occurred in patients with metastatic tumors from pancreatic cancer within 1 wk after RFA. The tumors were located near the liver surface with the size of 3.5 and 4.5 cm. One of them recovered after 2-3 mo of percutaneous drainage but the other did not till death.
Skin burn occurred in one case. The patient had large scars in the abdominal wall due to previous operation for purulent cholecystitis. It was difficult to avoid the scar area when we inserted the RFA electrode to treat a 5-cm tumor in segment IV. The outer insulate coat of the electrode melted during the RFA and led to skin burn of the scarred area. Thus it was essential to avoid the scar while inserting the electrode.
Besides these major complications, other patients complained about some mild complications, such as fever and bellyache. The temperature could reach about 37.8-38.5 °C, and lasted about several days to two weeks. If the temperature was too high or lasted too long, a blood test should be taken in time and relevant treatments should be adopted. The anesthetic drugs used during the RFA process could cause vomiting and nausea. The symptoms of bellyache post-RFA could be relieved within 3-7 d without any drugs; however, little acesodyne was occasionally needed. But if the tumor was close to the diaphragm, the RFA could induce an ache on the right shoulder; besides, if the intercostals nerve, peritoneum, or abdominal wall were injured, a transient breath-related pain could happen, and sometimes acesodyne was needed.
Some patients experienced transient liver dysfunction 2 wk post RFA; Western or Chinese medicines to protect liver function should be adopted to facilitate the general health and liver function.
It was easy and feasible to perform RFA on liver tumors. Most RFA could be administered with local anesthesia and acesodynes on outpatient, and the rest of the patients could be discharged after 1-2 d of observation. The RITA electrode in our practice was satisfying, and the information about temperatures at the needle tip helped to control the RFA area. Another advantage was RFA could be performed repeatedly on the condition of subsequent metastases or tumor recurrence. The quality of patient life and the immune status was good after RFA treatment. One patient’s with HCC was repeatedly treated 11 times and 21 tumors in total were ablated successfully, the patient has survived for more than 4 years. Another patient with liver metastases from stomach sarcoma was treated seven times and 11 metastases in total were treated, the patient has survived for 27 mo. The obvious advantage was that the whole cost of RFA was less expensive than other treatment options; RFA was minimally invasive and relatively safe, most patients experienced body weight increase, improvement of appetite and general health without serious liver dysfunction after RFA. There were seven cases who had mild to severe ascites before RFA, the ascites of two patients disappeared and that of three decreased significantly after RFA, further supporting the advantages of RFA in its minimal invasiveness and good efficacy.
Most of the liver tumor patients in China were at the very late stages when they were diagnosed, and large tumors were commonly seen. In order to develop the RFA technique effectively, we should pay enough attention to the standard protocol and the operation skill. Combination treatments may help to improve the therapeutic efficacy, including hepatic artery embolization or infusion before RFA, combined resection and RFA[15,18] and subsequent stage II surgical resection after RFA. These options will no doubt improve the therapeutic level of liver tumors.
RFA, as a minimally invasive local treatment of liver tumors, was an effective and relatively safe alternative for the advanced liver tumor patients, tumor recurrence, liver metastases which were unresectable or difficult to treat with traditional therapies. The complications usually occurred during or shortly after RFA, so we should follow them up closely to find the possible hemorrhage or periphery structures injury in time; as long as the precautious measures are taken and effective managements are carried out, the serious complication rate would be reduced to the least, so that the final goal of improvement of general health and long-term survival time could be reached.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 805] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 600] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 4. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 740] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 5. | Chen MH, Liu JB, Yan K, Wu JY, Huo L, Yang RJ, Zhang XP, Huang XF. Ultrasound-guided radiofrequency ablation of malignant hepatic tumors. Chin J Ultrasonogr. 2001;10:404-407. |
| 6. | Cai ZH, Chen T, Chen Q, Wang T, Shi L. The ultrasound-guided method for radio-frequency ablation to treat large hepatocelluar carcinoma. Chin J Ultrasonogr. 2001;10:408-409. |
| 7. | Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, Dai Y. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Chen MH, Yan K, Wu JY, Yang W, Dai Y, Song YP, Huang XF. Ultrasound-guided radiofrequency ablation for the treatment of 131 patients with malignant hepatic tumors. Chin J Gen Surg. 2002;17:520-522. |
| 9. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 878] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 10. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma & lt; or =4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67-76. [PubMed] |
| 12. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 390] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 13. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 931] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 14. | Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 259] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Buscarini L, Buscarini E, Di Stasi M, Quaretti P, Zangrandi A. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med. 1999;20:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Wu JY, Chen MH, Yan K, Zhang H, Wang HY, Huo L, Yang W, Song YP. Ultrasound-guided radiofrequency ablation in the treatment of liver metastases. J Peking University. 2001;33:449-451. |
| 17. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 263] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |