Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6385
Revised: February 15, 2005
Accepted: February 18, 2005
Published online: October 28, 2005
AIM: To investigate the effect and mechanism of action of the nitric oxide synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME) on invasion and metastasis of human colorectal cancer cell line SL-174T.
METHODS: Human colorectal cancer cell line SL-174T was cultured and treated separately with four different dosages of L-NAME for 72 h. Nitric oxide (NO) production was measured with Griess reagent. The effect of L-NAME on invasion and migration of SL-174T cells were evaluated by using Transwell chambers attached with polycarbonate filters and reconstituted basement membrane (Matrigel). RT-PCR was performed to determine the mRNA levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitor metalloproteinase-2 (TIMP-2).
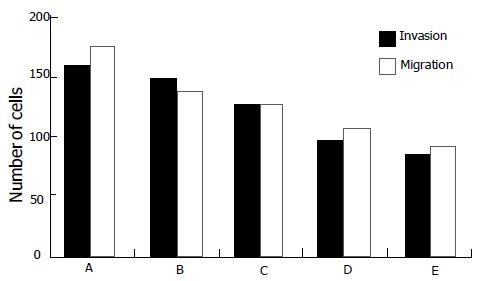
RESULTS: L-NAME could significantly inhibit NO production of SL-174T in a dose-dependent manner. After being treated for 72 h with 0.2, 0.4, 0.8, and 1.0 mmol/L L-NAME, respectively, the ability of the L-NAME treated SL-174T cells to invade the reconstituted basement membrane decreased significantly (t = 8.056, P<0.05; t = 14.467, P<0.01; t = 27.785, P<0.01; and t = 29.405, P<0.01, respectively) and the inhibition rates were 10.29%, 19.62%, 34.08%, and 42.23%, respectively. Moreover, L-NAME could inhibit migration of SL-174T cells, and the inhibition rates were 20.76%, 24.95%, 39.43%, and 46.85% for L-NAME at 0.2, 0.4, 0.8, and 1.0 mmol/L, respectively (t = 15.116, P<0.01). In addition, after treatment with L-NAME, expression of MMP-2 mRNA was significantly decreased (t = 71.238, P<0.01) and that of TIMP-2 mRNA was markedly increased (t = -13.020, P<0.01).
CONCLUSION: L-NAME exerts anti-invasive and anti-metastatic effects on SL-174T cell line via downregulating MMP-2 mRNA expression and upregulating TIMP-2 mRNA expression.
- Citation: Yu LB, Dong XS, Sun WZ, Zhao DL, Yang Y. Effect of a nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on invasion of human colorectal cancer cell line SL-174T. World J Gastroenterol 2005; 11(40): 6385-6388
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6385.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6385
Colorectal cancer is one of the leading causes of cancer deaths in men and women, and more effective and potent agents for chemotherapy of colorectal cancer are urgently needed. A number of in vitro and in vivo investigations have indicated the possible involvement of nitric oxide (NO) in promoting tumor carcinogenesis, migration, invasiveness, and angiogenesis. NO is produced from L-arginine, catalyzed by three isoforms of nitric oxide synthases (NOS): (1) calmodulin- and Ca2+-dependent endothelial NOS (eNOS); (2) neuronal NOS (nNOS); and (3) calmodulin- and Ca2+-independent endothelial NOS (iNOS)[1]. An NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME) has been reported to exert anticancer effects against human head and neck cancer and mammary tumor in vitro[2,3]. However, the effects of this agent against colorectal cancer remain to be determined. In the present study, we, therefore, examined the anticancer effects and mechanisms of L-NAME in highly metastatic colorectal cancer cell line SL-174T.
The human colorectal cancer cell line SL-174T was purchased from Cancer Research Institute of Harbin Medical University and maintained in RPMI 1640 medium (Gibco/BRL), supplemented with 100 mL/L heat-inactivated fetal calf serum (Gibco) and 10 g/L penicillin-streptomycin solution (10 000 U/mL). The cells were incubated in plastic tissue culture flasks (Falcon) at 37 °C in a humidified atmosphere containing 50 mL/L CO2 in air. In the experiments designed to block NO synthesis, L-NAME (Sigma) was added to the medium at various concentrations (0.2, 0.4, 0.8, and 1.0 mmol/L).
Using a microplate assay with Griess reagent, NO production was determined by measuring nitrite accumulation in culture supernatants. After the cells were treated with different concentrations of L-NAME (0.2, 0.4, 0.8, and 1.0 mmol/L) for 72 h, NOS activity was assessed by measuring nitrite production following the manufacturer’s instructions. Cell supernatants (1 000 μL) were mixed with 1 000 μL of the Griess reagent. After 10-min incubation at room temperature, the absorbency was read at 540 nm on a spectrophotometer.
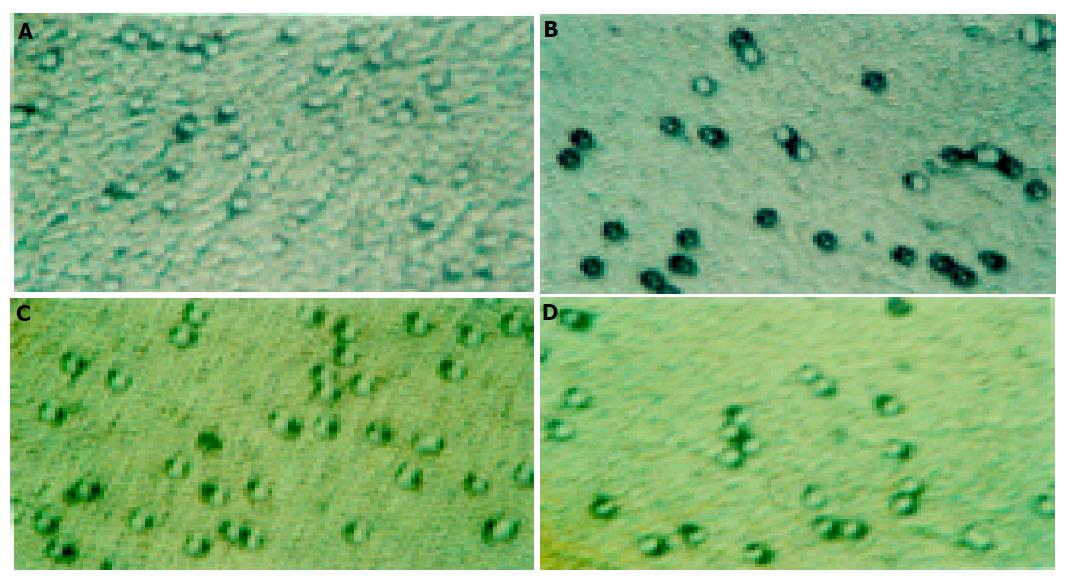
The ability of cells to migrate through control (migration) or invade through Matrigel-coated filters (invasion) was measured in a Transwell chamber (Costar). Eight-micron Transwell filters, which were coated with or without 5 μg Matrigel (Becton Dickinson) and 5 μg fibroblast (Promega), were placed in the lower chamber as a chemoattractant. Then SL-174T cells, treated with different concentrations of L-NAME for 72 h, were added into the upper room and incubated for additional 4 h at 37 °C in a humidified atmosphere containing 50 mL/L CO2 in air. The cells that had not penetrated the filter were wiped out with cotton swabs, and the cells that had migrated to the lower surface of the filter were examined using bright field microscopy, and photographed. The values for invasion were determined as the average number of migrated cells bound per microscopic field over four fields per assay and expressed as average values for triplicate experiments.
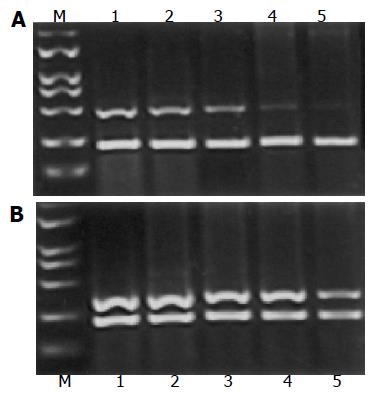
After the cells were treated with different concentrations of L-NAME (0.2, 0.4, 0.8, and 1.0 mmol/L) for 72 h, total RNA was extracted from SL-174T cells with the TRIzol reagent (Gibco/BRL, Life Technologies Inc.) according to the manufacturer抯 instructions. The primers were designed using Primer Premier 5 software. The primers used for PCR amplification of matrix metalloproteinase-2 (MMP-2) were 5’-TCTTCAAGGACCGGTTCATTTG-3’(sense) and 5’-GATGCTTCCAAACTTCACGCTC-3’(antisense), 500 bp long. The primers used for PCR amplification of tissue inhibitor metalloproteinase-2 (TIMP-2) were 5’-ACC TGGATGCCGTCGTGGAC-3’(sense) and 5’-TGTGGCAGCACCAGGGCAGC-3’(antisense), 376 bp long. The primers used for β-actin PCR amplification were 5’-TACATGGCTGGGGTGTTG AA-3’(sense) and 5’-AAGAGAGGCATCCTCACCCT-3’(antisense), 218 bp long. PCR primers were synthesized by Biology Engineering Corporation of Shanghai. Ten-microliter aliquots of the PCR product were loaded on 20 g/L polyacrylamide gel. Absorbency ratios of MMP-2 and TIMP-2 to control β-actin mRNA expression were considered as expression indices of the former two genes and used for semi-quantitation.
SAS for Windows 6.12 was used to solve the data. The data were mean value of at least three different experiments and expressed as mean±SD. The LSD-t test and Dunnett’s t test were used to compare the data. P<0.05 was considered statistically significant.
NO production was significantly decreased after the treatment with different dilutions of L-NAME. We observed significant differences in NO levels between control group and treatment groups (Table 1).
| Concentration mmol/L | No. of experiments | NO (μmol/L) 72 h |
| Control | 3 | 147.80±0.78b |
| 0.2 | 3 | 135.66±0.45 |
| 0.4 | 3 | 95.73±0.23 |
| 0.8 | 3 | 59.80±0.65 |
| 1 | 3 | 56.43±0.64 |
Compared with the control group, SL-174T cells treated with different concentrations of L-NAME for 72 h had decreasing tendency for invasion and the inhibition rates were 10.29%, 19.62%, 34.08%, and 42.23% for L-NAME at 0.2 mmol/L (P<0.05), 0.4, 0.8, and 1.0 mmol/L (P<0.01), respectively. In addition, L-NAME could markedly inhibit SL-174T cells migration, and the inhibition rates were 20.76%, 24.95%, 39.43%, and 46.85% for L-NAME at 0.2, 0.4, 0.8, and 1.0 mmol/L (P<0.01), respectively (Figures 1 and 2).
After the treatment with L-NAME for 72 h, expression of MMP-2 mRNA and TIMP-2 in SL-174T cells is shown in Figures 3A and B. The expression of MMP-2 mRNA was significantly decreased in the treatment groups as compared to the control (P<0.05). On the contrary, the expression of TIMP-2 mRNA was significantly higher in the treatment groups as compared to the control (P<0.05).
NO has dual action on tumor growth that may be dependent on local concentrations, type of tumor, and susceptibility of tumor cells to NO. Over production of endogenous NO would cause tumor toxicity, induce cell apoptosis, and prevent tumor angiogenesis. NO level produced in tumor microenvironment usually was up to two orders of magnitude lower than that associated with NO-dependent tumor apoptosis. Endogenous NO produced by blood vessel endothelial cells and tumor cells plays an important role in supplying tumor blood and maintaining tumor growth, also in dilating blood vessel and increasing angiogenesis[4,5]. NO can dilate arteriola, decrease leukocyte-endothelial interactions, and enhance vascular permeability in solid tumor. Much scientific research has focused on the role of NO in tumor progression; although two apparently conflicting views exist, overall an overwhelming amount of clinical and experimental evidence supports a positive association between NO production and tumor progression. The level of NOS protein and/or activity in the tumor has been shown to be positively correlated with the degree of malignancy for tumors of the human reproductive tract[6], breast[7,8], central nervous system[5], lung cancer[10], and prostate carcinomas[11]. Several studies have shown an increase of iNOS expression in colorectal cancer tissue when compared to normal mucosa and its extent correlates with tumor metastasis[12,13]. A specific cause-effect relationship between the upregulation of the iNOS gene and colon cancer has been recently demonstrated by Ahn and Oshima[14]. It has been demonstrated that tumors derived from human colon adenocarcinoma cells engineered to generate NO continuously (iNOS-19) are significantly more vascularized and more invasive than the wide controls.
This study clearly demonstrated that human colorectal cancer cell line SL-174T exhibited a strong ability to invade Matrigel and the NOS inhibitor L-NAME markedly decreased the invasion and migration. We explored the mediators of the NO-stimulated invasion, which lead to essential steps as migration and matrix degradation. MMPs and TIMPs have the ability to modulate the structure of the extracellular matrix (ECM), and changes of their expression level or activation of MMPs weaken the integrity of the ECM, which is a prerequisite for invasion and metastasis. Our study demonstrated that NOS inhibitor L-NAME reduced invasive and migratory capacities of cell line SL-174T by downregulation of MMP-2 mRNA and upregulation of TIMP-2 mRNA expression. Further work needs to demonstrate a corresponding shift in the activities of the enzyme and its inhibitors at the protein level. Additional mechanisms underlying NO-mediated stimulation of cellular invasiveness may exist. For example, NO has been shown to upregulate urokinase-type plasminogen activator (uPA) in endothelial cells of post-capillary venules during NO-mediated stimulation of angiogenesis[15]. Since uPA converts plasminogen to plasmin, which can activate numerous MMPs, this may represent another pathway of NO-mediated stimulation of matrix degradation.
In conclusion, the NOS inhibitor L-NAME can reduce invasive and migratory capacities of SL-174T cell line via downregulating MMP-2 mRNA expression and upregulating TIMP-2 mRNA expression. Thus, NOS inhibitors may prove to be important components of combination therapy protocols in human tumors, including colorectal cancer.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Geller DA, Billiar TR. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998;17:7-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 234] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Gallo O, Masini E, Morbidelli L, Franchi A, Fini-Storchi I, Vergari WA, Ziche M. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst. 1998;90:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 316] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Jadeski LC, Lala PK. Nitric oxide synthase inhibition by NG-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol. 1999;155:1381-1390. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] |
| 5. | Fukumura D, Jain RK. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev. 1998;17:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994;54:1352-1354. [PubMed] |
| 7. | Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 432] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Dueñas-Gonzalez A, Isales CM, del Mar Abad-Hernandez M, Gonzalez-Sarmiento R, Sangueza O, Rodriguez-Commes J. Expression of inducible nitric oxide synthase in breast cancer correlates with metastatic disease. Mod Pathol. 1997;10:645-649. [PubMed] |
| 9. | Cobbs CS, Brenman JE, Aldape KD, Bredt DS, Israel MA. Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res. 1995;55:727-730. [PubMed] |
| 10. | Fujimoto H, Ando Y, Yamashita T, Terazaki H, Tanaka Y, Sasaki J, Matsumoto M, Suga M, Ando M. Nitric oxide synthase activity in human lung cancer. Jpn J Cancer Res. 1997;88:1190-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Kojima M, Morisaki T, Tsukahara Y, Uchiyama A, Matsunari Y, Mibu R, Tanaka M. Nitric oxide synthase expression and nitric oxide production in human colon carcinoma tissue. J Surg Oncol. 1999;70:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Lagares-Garcia JA, Moore RA, Collier B, Heggere M, Diaz F, Qian F. Nitric oxide synthase as a marker in colorectal carcinoma. Am Surg. 2001;67:709-713. [PubMed] |
| 14. | Ahn B, Ohshima H. Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res. 2001;61:8357-8360. [PubMed] |
| 15. | Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 629] [Article Influence: 20.3] [Reference Citation Analysis (0)] |