Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6354
Revised: April 1, 2005
Accepted: April 2, 2005
Published online: October 28, 2005
AIM: To evaluate the imaging findings of biliary hamartomas (von Meyenburg complexes, VMCs) and discuss the differential diagnosis with other related diseases.
METHODS: Imaging findings of biliary hamartomas on ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), MR cholangiopancreatography (MRCP)and hepatobiliary scintigraphy were retrospectively analyzed in six patients.
RESULTS: On ultrasound images, five of the six cases showed multiple small hyper- and hypo-echoic lesions with comet-tail echoes, especially when magnified by US with the usage of zoom function. In all the six cases, multiple tiny hypodense lesions less than 10 mm in diameter were revealed as scattered throughout the liver with no enhancement on CT. These tiny lesions were demonstrated to be hyper- and hypo-intensity on T2- and TI-weighed images, respectively, in three patients who underwent MRI examinations. MRCP was performed in two patients, and clearly showed multiple tiny irregular- and round-shaped hyper-intensity lesions. MRCP and hepatobiliary scintigraphy showed normal appearances of intra- and extra-hepatic bile ducts in two and one patients, respectively.
CONCLUSION: Imaging modalities are useful in the diagnosis and differential diagnosis of VMCs. A correct diagnosis might be obtained when typical imaging findings are present even without a histological confirmation.
- Citation: Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol 2005; 11(40): 6354-6359
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6354
Biliary hamartomas also called as von Meyenburg complexes (VMCs) are benign liver malformations that histologically contain cystic dilated bile ducts within 10 mm in diameter surrounded by abundant fibrous stroma[1,2]. They are usually uncovered by autopsy as an incidental finding. Detecting by imaging modalities is thought to be uneasy because of their asymptomatic nature and small size[3]. To our knowledge, except for a few case reports, there are only two imaging studies on VMCs with small series of cases that have been published in the literature so far[1,3]. Although VMCs are rare, they are easily confused with metastatic diseases of the liver on imaging[4]. Therefore, discerning the imaging characteristics of VMCs is desirable for the differential diagnosis, and thus reducing the needs for invasive methods such as biopsy or laparotomy[5].
Herein, we retrospectively analyze the imaging findings of VMCs on ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), MR cholangiopancreatography (MRCP), and hepatobiliary scintigraphy in six patients, and discuss the differential diagnosis with other related diseases.
There were four men and two women with ages ranging from 29 to 72 years (mean, 54 years). Hepatic lesions were found incidentally when the patients underwent routine physical checkup by US (n=2), screening for liver metastasis of a known ovary carcinoma by US and CT (n=1), and abdominal US examinations for Hashimoto disease associated with abnormal liver function tests (n=1), as well as diabetes (n=2). All imaging studies including US, CT, and MRI were performed in three patients. Among them, additional MRCP was performed in two patients; hepatobiliary scintigraphy was carried out in another patient. Both US and CT scans were available in another three patients. Three- to ten-time follow-up US and/or CT examinations were obtained in all the six patients for over a period of 7-100 months. Histologic confirmation was acquired in three patients.
US examinations were performed by using GE LOGIQ 700 EXPERT series and LOGIQ 500 MD series (GE Medical System, Milwaukee, WI, USA), Toshiba Powervision 8000 (Toshiba Medical System) with convex probes at the frequency of 3.0-4.4 MHz. A helical CT system (Toshiba X-vigor) was used for CT scans. The section thickness was 7 mm with no interslice gap. Both plain and enhanced CT scans were carried out in five patients. For enhanced CT, a total of 100 mL of Iopamiron (Iopamidol, Nihon Schering) was intravenously injected with the iodine concentration of 370 mg/mL. MRI was performed by using a 1.5 T MR unit (VISART HYPER, Toshiba) with 160×256 matrix. SE T1- and T2-weighed images were acquired by using 500/15 ms (TR/TE) and 3 000/80 ms (TR/TE), respectively, with two excitations. Slice thickness was set to 8 mm, and interslice gap of 1 mm. Fast SE MRCP was performed with 6 000/250 ms (TR/TE), and 4-8 mm slice thickness. Hepatobiliary scintigraphy was carried out by using 99mTC-N-pyridoxy 1-5-methyl tryptophan (99mTC-PMT).
Multiple hepatic lesions were scattered throughout both the right and left liver lobes in five patients, distributed predominantly in the right liver lobe in one case. Of the five patients with scattered distribution of the lesions, apparent location of the lesions in the subcapsular areas was observed in four cases. The lesion size measured within 10 mm in five cases. In another case, most of the lesions were less than 10 mm in diameter; in addition, four to five typical cysts with round or oval shape in the right liver measuring from 11 to 25 mm were noted in one case. Multiple right renal cysts (3 in number and 10-20 mm in diameter) together with one cyst (10 mm in diameter) in the left kidney were detected in one case.
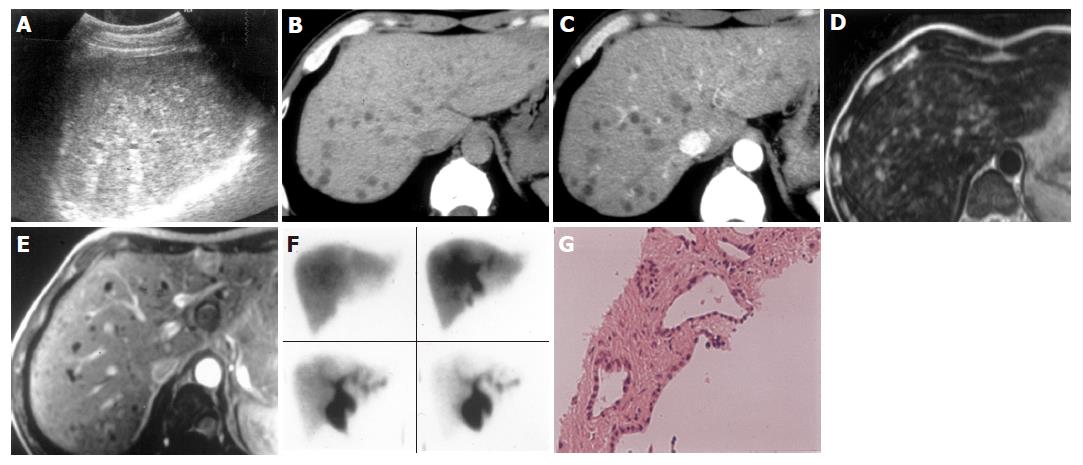
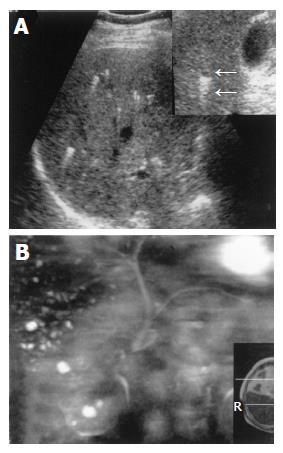
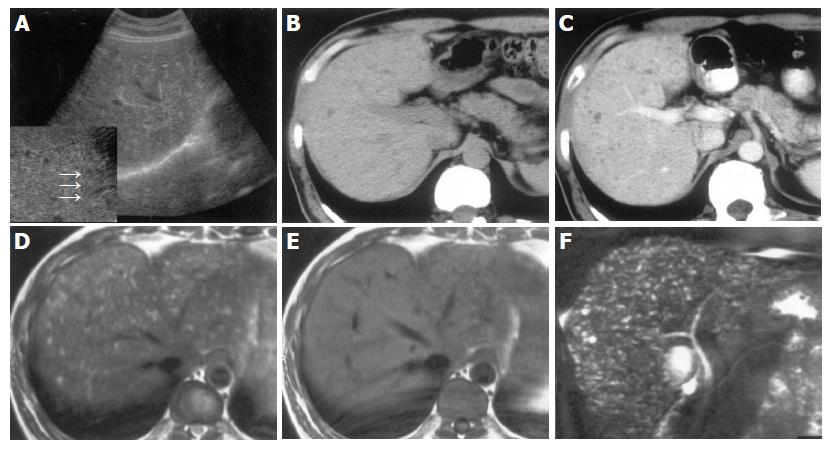
On US images, five cases showed multiple small hyper- and hypo-echoic lesions with comet-tail echoes (Figure 1A). Liver echo textures were heterogeneous. When zoom function was used, some small hyper-echoic dot-like lesions were showed to be tiny cystic lesions with distal acoustic enhancement (comet-tail echoes) (Figures 2A and 3A). One case was revealed as multiple hypo-echoic lesions. In five cases, three- to ten-time follow-up US examinations were performed for 19-100 mo, and no remarkable changes were found.
On plain CT, multiple hypodense lesions with irregular and round shape were observed in six cases (Figures 1B and 3B). After intravenous administration of contrast medium, all the lesions showed no enhancement in either the periphery or the center areas, and the delineation of all the lesions became more conspicuous than on plain CT (Figures 1C and 3C). Three- to six-time follow-up CT scans performed for 7-84 months in three patients revealed no obvious changes of the lesions when compared with the findings on the first time CT images.
MRI was performed in three cases. Multiple small hepatic lesions were demonstrated to be of low signal intensity on T1-weighed images and high signal intensity on T2-weighed images (Figures 1D, 3D, and E). After gadolinium administration was performed in one case, no enhancement was noted on T1-weighed images (Figure 1E). In two cases, MRCP clearly portrayed numerous tiny irregular-shaped and few round-shaped small hyper-intense nodules (Figures 2B and 3F). The lesions were displayed more distinctly and in more numbers on MRCP than on CT and MRI (Figure 3). Intra- and extra-hepatic bile ducts were normal (Figures 2B and 3F). Hepatobiliary scintigraphy performed in one case showed no obvious pooling in the liver, which was found in Caroli’s disease. The biliary system excreting function of radioisotope was normal (Figure 1F).
US guided liver biopsy was performed in three cases. On histology, multiple dilated bile ducts lined by a single layer of cubic epithelium (Figure 1G) were revealed in two cases, and the diagnosis of VMCs was confirmed. The patient with ovary carcinoma underwent operations twice for hysterectomy plus ovariotomy, and secondary omentectomy, respectively. During the operation, liver metastasis was suspected by the palpation of the liver surface. However, US and CT displayed multiple small typical cystic lesions without any changes on three-time follow-up CT scans performed for over 7 mo period. US guided liver biopsy was performed without demonstrating any malignant cells, although VMC findings were also not found. Liver metastases were not considered according to imaging findings. VMCs were thought to be the most possible diagnosis. The patient died from severe complications after secondary omentectomy later.
The details of the six cases with VMCs are sum-marized in Table 1.
| No | Sex/age( yr) | ClinicalBackground | Imaging findings | Histologicaldiagnosis | ||||||||||
| Size (mm) | Number | Distribution | US | CT | MRI | MRCP/scintigraphy | Associatedfindings | Follow-up.(time: month) | ||||||
| Attenuation | enhancement | T1 | T2 | |||||||||||
| 1 | M/51 | No symptoms | <10 | Multiple | Scattered, whole liver | Hyper- and hypoechoic, with comet-tail echoes | Low | No | Hypo-intensit y | Hyper-intensity | Normal bile ducts | No Hepatic cysts | US for 52 mo, no change CT for 7 mo, | No |
| 2 | F/61 | Ovary carcinoma | most lesions <10, several cysts 11-25 | Multiple | Scattered, whole liver | Hyper- and hypoechoic, with comet-tail echoes | Low | No | no change | |||||
| 3 | F/68 | Hashimoto disease with liver damage | <10 | Multiple | Scattered, whole liver | Hypoechoic | Low | No | No | US/CT for 19–48 mo, no change | ||||
| 4 | M/72 | No symptoms | <10 | Multiple | Predominant in the right lobe | Hyper- and hypoechoic, with comet-tail echoes | Low | No | Hypo-intensity | Hyper-intensity | Hyperintensity, normal bile ducts(MRCP) | Right and left renal cysts | US/C T for 27–84 mo, no change | |
| 5 | M/44 | Diabetes | <10 | Multiple | Scattered, whole liver | Hyper- and hypoechoic, with comet-tail echoes | Low | No | Hypo-intensity | Hyper-intensity | Hyperintensity, normal bile ducts (MRCP) | No | US for 25 mo, no change | VMCs |
| 6 | M/29 | Diabetes | <10 | Multiple | Scattered, whole liver | Hyper- and hypoechoic, with comet-tail echoes | Low | No | No | US for 100 mo, no change | ||||
VMCs are considered as congenital bile duct mal-formations due to the failure of embryonic involution[2,4,6]. Macroscopically, the lesions present as gray-white to gray-yellow or black nodules mostly uniform in size measuring less than 5 mm, some up to 10 mm in diameter[3,7,8]. They are usually scattered throughout both of the liver lobes, especially in the subcapsular region of the liver[8]. Microscopically, VMCs are generally well defined, irregular or round in shape, consisting of a variable number of dilated, tortuous or branching bile ducts that were lined by a single layer of cuboidal cells and embedded in a fibrocollagenous stroma[4,8,9]. The lumina of these dilated bile ducts sometimes contain bile-stained granular or amorphous materials[4,8]. Some VMCs contain sclerotic arteries, however, most of them are not associated with vascular proliferation, and some are even devoid of vessels[4,8]. The incidence of VMCs was estimated at 0.69-5.6% in autopsy series[8,10], and 0.6% in needle biopsy series[9]. In some cases, association with polycystic liver and kidney diseases, simple liver cysts or pancreatic cysts were reported in the literature[2-4,6,7,10]. Although VMCs are generally considered to be benign liver lesions without clinical manifestations, less frequent association with malignant transformation was also described[11,12]. However, the most important clinical significance is that VMCs are easily misdiagnosed as multiple liver metastases on imaging[2,6,13,14] or even on gross examination[8].
Imaging manifestations of VMCs are various[2-6,14-17] and have not been well illustrated yet. US findings have been described as hypoechoic, hyperechoic or mixed heterogenic echoic structures[6,7,13-15]. These variations might reflect the histologic features of VMCs including dilated bile ducts and fibrocollagenous stroma. Luo et al[3] described the sign of multiple comet-tail echoes, and speculated that it might be the specific US finding of VMCs. However, this sign was observed only in one case of their study. In our series, multiple small comet-tail echoes appeared in all but one case. It manifested as posterior echo enhancement of the lesions, which might be due to the cystic feature of dilated bile duct and therefore resulted in good transmission of the sound beam. Especially when magnified by US, some small hyperechoic lesions were actually found to be tiny cystic lesions with comet-tail echoes. This evidence strongly suggests that the sign of multiple small comet-tail echoes is a unique US feature of VMCs, which have diagnostic value.
On plain CT images, almost all VMCs that had been reported were demonstrated to be multiple small hypodense lesions[1-5,7,13,14,16,17]. While on enhanced CT images, although homogeneous enhancement of the lesions was noted in two case reports[2,17], no enhancement of the lesions was observed in most of the reported cases after intravenous administration of contrast medium[1,3-5,13,14,16], as in our series. This phenomenon might correlate to the poor vascularity of VMCs described on histology[4].
On MRI, VMCs, including our series, were revealed as hypo-intense on T1-weighed images and hyper-intense on T2-weighed images when compared with surrounding liver parenchyma[3,5,7,17-19]. Recently, MRCP has been considered to be highly sensitive in depicting intra- and extrahepatic bile duct anomalies and cystic lesions of the liver as well as their relationship with bile duct system[5,20], which is superior to the sensitivity of CT[20]. In the two cases of our study, MRCP displayed the VMC lesions more clearly than CT and MRI concerning both the lesion number and shape. As for the superior sensitivity of MRCP, this may be partially due to the relatively large slice thickness used in CT and MRI scans in our study, resulting in overlooking of lesions smaller than the slice thickness. In addition, the poor MR imaging quality in our study also accounts for one of the reasons.
Some authors claimed that imaging findings of VMCs were not specific and liver biopsy was needed for a definitive diagnosis[1,4]. However, with the use of advanced imaging modalities and long-term imaging follow-up, some authors pointed out that it might be possible to make a correct diagnosis of VMCs by imaging[4,5]. This view is supported by our studies. When typical imaging findings appear, such as multiple small comet-tail echoes on US, multiple tiny hypodense lesions scattered throughout the liver with no enhancement on CT, and cystic appearance with normal extra- and intrahepatic bile duct on MRI and MRCP, a diagnosis of VMCs can be considered. Liver biopsy may have limitations such as sample errors and performance difficulties due to the very small size of the VMCs, which was reflected by the fact that the reported incidence (0.6%)[9] of VMCs in a series of needle biopsy was relatively lower than that of autopsy (0.69-5.6%)[8,10]. Therefore, long-term follow-up by imaging examinations may have the same important significance in the diagnosis of VMCs. Although histological confirmation was obtained in only three cases in our series, typical imaging findings and relative long-time imaging follow-up that showed identical findings are strongly suggestive of VMCs. However, the case number in our series is small. Further observations on large series are still needed to clarify our stand.
The spectrum of differential diagnosis of VMCs is fairly wide. However, the most important one is liver metastasis especially in patients with extrahepatic malignant tumors. Usually, multiple small metastases are ill defined on plain CT, and show various degrees of enhancement (such as rim enhancement) after intravenous administration of contrast medium. However, for difficult patients, final exclusion of metastatic lesions should still depend on liver biopsy or follow-up imaging studies. Diffuse primary hepatocellular carcinoma usually occurs in cirrhotic patients, and is seldom revealed to be a cystic lesion on US and CT. Simple hepatic cysts are variable in number, size, and location, and usually round in shape[3]. As VMCs may coexist with simple hepatic cysts or polycystic liver and kidney diseases[2-4,6,7,10], sometimes it is difficult to make a definitive differentiation especially from polycystic liver disease on imaging. We are likely to approve simple hepatic cysts, when the lesions are larger than 10 mm in diameter and round in shape, as in one case of our series, since most VMCs were reported to be less than 10 mm in diameter[1-5]. Peribiliary cysts are multiple small cystic dilatations of the intrahepatic extramural peribiliary glands[21] and should also be included in the differential diagnoses of VMCs. However, they are located exclusively in the hepatic hilum and along the larger portal tract[21], which is different from the scattered distribution of VMCs. In addition, some peribiliary cysts were reported to be gradually increasing in size and number[20], which has never been described in VMCs. Microabscesses of the liver can be differentiated from VMCs by means of clinical and radiological data, such as having a history of immuosuppression, symptoms of fever and epigastralgia, multiple round or loculated hypodense lesions on CT[4,16,22], and “target” appearance on US[22]. Furthermore, intrahepatic bile duct anomalies such as dilated bile ducts and Caroli’s disease can be readily distinguished from VMCs by imaging, especially when MRCP is performed for this purpose[5], as MRCP offers optimal visualization of the spatial relationship between hepatic lesions and intrahepatic bile ducts[5,20]. Besides, biliary scintigraphy also facilitates the differentiation.
In conclusion, imaging modalities are useful in the diagnosis and differential diagnosis of VMCs. Imaging findings, such as multiple small comet-tail echoes on US, multiple tiny hypodense lesions scattered throughout the liver with no enhancement on CT, and cystic nature with normal extra- and intrahepatic bile duct on MRI and MRCP, can be considered as typical or highly suggestive manifestations of VMCs. A correct diagnosis might be obtained when typical imaging findings are present even without a histological confirmation. However, in patients with extrahepatic malignant tumors, follow-up imaging examinations or liver biopsy are needed.
Science Editor Ma JY and Guo SY Language Editor Elsevier HK
| 1. | Lev-Toaff AS, Bach AM, Wechsler RJ, Hilpert PL, Gatalica Z, Rubin R. The radiologic and pathologic spectrum of biliary hamartomas. AJR Am J Roentgenol. 1995;165:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Wei SC, Huang GT, Chen CH, Sheu JC, Tsang YM, Hsu HC, Chen DS. Bile duct hamartomas. A report of two cases. J Clin Gastroenterol. 1997;25:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Luo TY, Itai Y, Eguchi N, Kurosaki Y, Onaya H, Ahmadi Y, Niitsu M, Tsunoda HS. Von Meyenburg complexes of the liver: imaging findings. J Comput Assist Tomogr. 1998;22:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 4. | Cooke JC, Cooke DA. The appearances of multiple biliary hamartomas of the liver (von Meyenberg complexes) on computed tomography. Clin Radiol. 1987;38:101-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Mortelé B, Mortelé K, Seynaeve P, Vandevelde D, Kunnen M, Ros PR. Hepatic bile duct hamartomas (von Meyenburg Complexes): MR and MR cholangiography findings. J Comput Assist Tomogr. 2002;26:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Saló J, Bru C, Vilella A, Ginès P, Gilabert R, Castells A, Bruguera M, Rodés J. Bile-duct hamartomas presenting as multiple focal lesions on hepatic ultrasonography. Am J Gastroenterol. 1992;87:221-223. [PubMed] |
| 7. | Gallego JC, Suarez I, Soler R. Multiple bile duct hamartomas: US, CT, and MR findings. A case report. Acta Radiol. 1995;36:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Chung EB. Multiple bile-duct hamartomas. Cancer. 1970;26:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Thommesen N. Biliary hamartomas (von Meyenburg complexes) in liver needle biopsies. Acta Pathol Microbiol Scand A. 1978;86:93-99. [PubMed] |
| 10. | Redston MS, Wanless IR. The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies [corrected]. Mod Pathol. 1996;9:233-237. [PubMed] |
| 11. | Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, Fukamachi S, Hirohashi S. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch. 1995;426:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Papadogiannakis N, Gad A, Sjöstedt S, Tour R, Thörne A, Seensalu R. Adenocarcinoid of the liver arising within an area of hamartoma with predominant bile duct component. J Clin Gastroenterol. 1996;23:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Eisenberg D, Hurwitz L, Yu AC. CT and sonography of multiple bile-duct hamartomas simulating malignant liver disease (case report). AJR Am J Roentgenol. 1986;147:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Iha H, Nakashima Y, Fukukura Y, Tanaka M, Wada Y, Takazawa T, Nakashima O, Kojiro M. Biliary hamartomas simulating multiple hepatic metastasis on imaging findings. Kurume Med J. 1996;43:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Tan A, Shen JF, Hecht AH. Sonogram of multiple bile duct hamartomas. J Clin Ultrasound. 1989;17:667-669. [PubMed] [DOI] [Full Text] |
| 16. | Sada PN, Ramakrishna B. Computed tomography of von Meyenburg complex simulating micro-abscesses. Australas Radiol. 1994;38:225-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Martinoli C, Cittadini G, Rollandi GA, Conzi R. Case report: imaging of bile duct hamartomas. Clin Radiol. 1992;45:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Slone HW, Bennett WF, Bova JG. MR findings of multiple biliary hamartomas. AJR Am J Roentgenol. 1993;161:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Maher MM, Dervan P, Keogh B, Murray JG. Bile duct hamartomas (von Meyenburg complexes): value of MR imaging in diagnosis. Abdom Imaging. 1999;24:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kudo M. Hepatic peribiliary cysts: clinically harmless disease with potential risk due to gradual increase in size and number. J Gastroenterol. 2001;36:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Nakanuma Y, Sasaki M, Terada T, Harada K. Intrahepatic peribiliary glands of humans. II. Pathological spectrum. J Gastroenterol Hepatol. 1994;9:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Callen PW, Filly RA, Marcus FS. Ultrasonography and computed tomography in the evaluation of hepatic microabscesses in the immunosuppressed patient. Radiology. 1980;136:433-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |