Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6322
Revised: April 26, 2005
Accepted: April 30, 2005
Published online: October 28, 2005
AIM: To determine the effect of pioglitazone, a specific peroxisome proliferator-activated receptor-γ (PPARγ) ligand, on the development of acute pancreatitis (AP) and on the expression of heat shock protein 70 (HSP70) in the pancreas.
METHODS: AP was induced in rats by subcutaneous infusion of cerulein for 5 h. Pancreatic blood flow was measured by laser Doppler flowmetry. Plasma lipase activity, interleukin-1β (IL-1β) and IL-10 were determined. Pancreatic weight and histology were evaluated and pancreatic DNA synthesis and blood flow as well as pancreatic mRNA for IL-1β and HSP70 were assessed in rats treated with pioglitazone alone or in combination with cerulein.
RESULTS: Pioglitazone administered (10-100 mg/kg i.g.) 30 min before cerulein, attenuated dose-dependently the pancreatic tissue damage in cerulein-induced pancreatitis (CIP) as demonstrated by the improvement of pancreatic histology, reduction in plasma lipase activity, plasma concentration of pro-inflammatory IL-1β and its gene expression in the pancreas and attenuation of the pancreatitis-evoked fall in pancreatic blood flow. CIP increased pancreatic HSP70 mRNA and protein expression in the pancreas and this effect was enhanced by pioglitazone treatment.
CONCLUSION: Pioglitazone attenuates CIP and the beneficial effect of this pioglitazone is multifactorial probably due to its anti-inflammatory activities, to the suppression of IL-1β and to the overexpression of HSP70. PPARγ ligands could represent a new therapeutic option in the treatment of AP.
- Citation: Konturek PC, Dembinski A, Warzecha Z, Burnat G, Ceranowicz P, Hahn EG, Dembinski M, Tomaszewska R, Konturek SJ. Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, protects pancreas against acute cerulein-induced pancreatitis. World J Gastroenterol 2005; 11(40): 6322-6329
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6322.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6322
Acute pancreatitis (AP) is associated with high morbidity and mortality. Approximately 25% of patients develop severe inflammation with pancreatic and peripancreatic fat necrosis that requires intensive care. AP is a multifactorial disease associated with the release of digestive enzymes to the pancreatic interstitium and to the systemic circulation as well as with the increased cytokine production and release, which can ultimately lead to deleterious local and systemic effects[1].
In general, the pathomechanism of AP is still poorly understood. Recently, the modulatory role of peroxisome proliferator-activated receptors (PPARs) has been proposed in the inflammatory response of different organs[2]. The PPAR family consists of at least three different isoforms; PPARα, PPARδ, and PPARγ[3]. PPARγ is predominantly detected in adipose tissue, but it is also expressed in other tissues, including pancreatic tissue. When activated by a specific ligand, PPARs heterodimerize with retinoid X receptor and then bind to peroxisome proliferator response element leading to the change in the transcription of target genes involved in lipid metabolism, glucose homeostasis, cell proliferation and differentiation, and inflammatory response. PPARs are activated by natural ligands such as fatty acids, eicosanoids, oxidized fatty acids, and by pharmacological compounds such as glitazones. Pioglitazone is a member of glitazones and it has been clinically used to increase the sensitivity to insulin[4].
Several reports have demonstrated an anti-inflammatory action of the specific ligands of PPARγ. Activators of PPARγ inhibit the generation of pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interleukin-6[5] and this effect seems to be dependent on the inhibition of nuclear factor-κB (NFκB) pathway[6].
Heat shock-proteins (HSPs) are a group of cytoprotective proteins that help to maintain the metabolic and structural integrity of cells. HSPs are produced in many cell types in response to a variety of stresses, including heat, free radicals, and toxins[7]. HSPs are highly conserved proteins and play essential functions in protein folding, transport, translocation, degradation, and assembly[8]. In the pancreas, HSPs have been shown to provide the protection against cerulein- and arginine-induced pancreatitis[9,10]. HSP70 (also referred as HSP72 or inducible HSP70) is perhaps the most well-studied member of HSPs. However, it is not known whether PPARγ ligands interact with HSP70 and what is the biological consequence of this potential interaction.
The aim of the present study was: (1) to determine the effect of pioglitazone, a specific PPARγ ligand, on the development of acute cerulein-induced pancreatitis (CIP); (2) to assess the effect of treatment with pioglitazone on pancreatic expression of HSP70; and (3) to evaluate the effect of pre-treatment with pioglitazone on pancreatic synthesis and plasma level of IL-1β.
Studies were performed on male Wistar rats weighing 150-200 g, in accordance with the experimental protocol approved by the Committee for Research and Animal Ethics of Jagiellonian University College of Medicine, Cracow, Poland. Animals were housed in cages under standard conditions, on a commercial pellet chow, at room temperature with a 12-h light-dark cycle. Prior to the start of experiments, rats were deprived of food, while drinking water was available ad libitum. AP was induced by cerulein that was diluted in saline and infused subcutaneously (s.c.) for 5 h at a dose 10 μg/kg/h as described earlier[11].
To study the effect of pioglitazone on the development of AP, the rats were divided into following groups: (1) rats infused with 0.9% vehicle saline s.c. for 5 h (control); (2) rats infused with 0.9% saline s.c. for 5 h and pretreated with pioglitazone (10-100 mg/kg i.g., 30 min before saline infusion); (3) rats infused with cerulein (10 μg/kg/h) s.c. for 5 h; and (4) rats infused with cerulein (10 μg/kg/h) s.c. for 5 h and pretreated with pioglitazone (10-100 mg/kg i.g., 30 min before cerulein infusion). Animals were anesthetized and killed after 5 h of saline or cerulein infusion. This particular dose of pioglitazone 50 mg/kg was used because, in our previous studies, similar dose was highly effective in speeding up of the healing rate of gastric ulcer[12].
Following the infusion of saline or cerulein, animals were anesthetized with ketamine (50 mg/kg i.p., Bioketan, Biowet, Gorzów, Poland) and the abdomen was opened. The pancreas was exposed for the measurement of the blood flow by laser Doppler flowmeter, using PeriFlux 4001 Master monitor (Perimed AB, Järfälla, Sweden) as described previously[13]. The pancreatic blood flow was presented as percent change from the value obtained in rats infused with saline.
Immediately after the measurement of pancreatic blood flow, the abdominal aorta was exposed and blood was taken for the determination of plasma lipase activity, and plasma IL-1β and IL-10 concentrations as described before[14].
Plasma lipase activity was determined with a Kodak Ectachem DT II System analyzer (Eastman Kodak Company, Rochester, NY, USA). The values of plasma lipase activity were expressed as units per liter.
The values of plasma IL-1β and IL-10 were measured in duplicate, using the Biosource Cytoscreen rat IL-1β and IL-10 kit based on a solid phase sandwich ELISA (BioSource International Inc., Camarillo, CA, USA) according to the manufacturer’s instructions. Concentrations were expressed as picogram per milliliter.
After the collection of blood samples, the pancreas was dissected out and divided into samples for analysis of pancreatic mRNA, pancreatic protein, measurement of pancreatic DNA synthesis, and histological examination. Samples of pancreatic tissue for histological examination were fixed in 40 g/L formaldehyde and stained with hematoxylin and eosin. The histologic grading of edema was made using a scale ranging from 0 to 3; 0 = no edema, 1 = interlobular edema, 2 = interlobular edema and moderate intralobular edema, and 3 = interlobular edema and severe intralobular edema. Leukocytic infiltration was graded from 0 (absent) to 3 for maximal alterations (diffuse infiltration in the entire pancreatic gland). Grading of vacuolization was based on the appropriate percentage of cell involved; 0 = absent, 1 = less than 25%, 2 = 25-50%, and 3 = more than 50%.
The rate of DNA synthesis in samples of pancreatic tissue was determined as described previously[15]. Briefly, the minced pancreatic tissue was incubated at 37 °C for 45 min in 2 mL of medium containing 8 μCi/mL of [3H]thymidine ([6-3H]thymidine, 20-30 Ci/mmoL; Institute for Research, Production and Application of Radioisotopes, Prague, Czech Republic). DNA concentration was determined by the Giles and Myers[16] procedure. The incorporation of mL [3H]thymidine into DNA was determined by counting 0.5 mL DNA-containing supernatant in a liquid scintillation system. DNA synthesis was expressed as [3H]thymidine disintegrations per minute per microgram DNA.
Samples of pancreatic tissue for reverse transcriptase-polymerase chain reaction (RT-PCR) were snap-frozen in liquid nitrogen, and then stored at -80 °C until the time of RNA extraction. Total RNA was extracted from the pancreatic samples using the extraction kit from Stratagene (Heidelberg, Germany). Following precipitation, RNA was resuspended in RNAse-free water and its concentration was estimated by absorbance at 260 nm wavelength. RNA samples were stored at -80 °C until analysis.
Single stranded cDNA was generated from 5 μg of total cellular RNA using Moloney murine leukemia virus reverse transcriptase (Stratagene, Heidelberg, Germany) and oligo-(dT)-primers (Stratagene, Heidelberg, Germany) in accordance with the procedure described by our group previously[17]. The resultant cDNA (2 μL) was amplified in 50 μL reaction volume containing 2 U Taq polymerase, dNTP (200 μmol/L each), 1.5 mmol/L MgCl2, 5 μL 10 PCR buffer (100 mmol/L KCl, 20 mmol/L Tris-HCl, pH 8.3) and specific primers used at a final concentration of 1 mmol/L (all reagents were obtained from Takara, Shiga, Japan). The reaction mixture was amplified in a DNA thermal cycler (Perkin-Elmer-Cetus, Norwalk, CT, USA) and the incubation and thermal cycling conditions were as follows: denaturation at 94 °C for 1 min, annealing at 60 °C for 45 s and extension at 72 °C for 2 min. The number of cycles was 28 for GAPDH, 30 for HSP70, and 33 for IL-1β. The nucleotide sequence of the primers for GAPDH, IL-1β, and HSP70 were based on the sequences of the published cDNAs as described previously[18,19]. The primer sequence for IL-1β was: sense 5’-GCT ACC TAT GTC TTG CCC GT-3’; antisense 5’-GAC CAT TGC TGT TTC CTA GG-3’. Primer pair for IL-1β produced a 543-bp product according to the cDNA sequence. The primer sequence for HSP70 was: sense 5’-GTG AAG ATC TGC GTC TGC TTG-3’; antisense 5’-TTTGACAACAGG CTGGTG AAC C-5’. The expected length of the product was 590 bp. GAPDH primers were same as previously published by our group[17]. All primers were synthesized by GIBCO BRL/Life Technologies (Eggenstein, Germany). PCR products were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. Location of a predicted product was confirmed by using 100-bp ladder (Takara, Shiga, Japan) as a standard size marker.
Shock frozen tissue from rat pancreas was homogenized in a lysis buffer (100 mmol/L Tris-HCl, pH 7.4, 15% glycerol, 2 mmol/L EDTA, 2% SDS, 100 mmol/L DDT) by the addition of 1:20 dilution of aprotinin and 1:50 dilution of 100 mmol/L PMSF. Insoluble material was removed by centrifugation at 12 000 g for 15 min. Approximately 50µg of total protein extracts were loaded on SDS-polyacrylamide gels and run 40 mA, followed by transfer on nitrocellulose membrane (Protran, Schleicher & Schuell, Germany) by electroblotting. Three percent of BSA (Sigma-Aldrich, Germany) in TBS/Tween-20 buffer (137 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.4, 0.1% Tween-20) was used to block filters for at least 1 h at room temperature. Specific primary antibody against HSP70 (mouse monoclonal, 1:200 dilution; Stressgen Biotechnologies Corp., Canada), or β-actin (mouse monoclonal, dilution 1:5 000; Sigma-Aldrich, Germany) was added to the membrane, followed by an anti-mouse-IgG HRP conjugated secondary antibody (dilution 1:20 000; Promega, USA) dissolved in 1% non-fat milk in TBS-Tween-20 buffer. Incubation of primary antibody was followed by washing thrice with TBS-Tween-20 buffer for 10 min. Incubation of the secondary antibody was followed by five washes for 10 min. Immunocomplexes were detected by the SuperSignal West Pico Chemiluminescent Kit (Pierce, USA). Thereafter, the developed membrane was exposed to an X-ray film (Kodak, Wiesbaden, Germany). Comparison between different treatment groups was made by determining the examined protein/β-actin ratio of the immunoreactive area by densitometry as described elsewhere[18].
Comparison of the difference between the mean values of various groups of experiments was made by analysis of variance and the Student’s t-test for unpaired data. A difference with a P value of less than 0.05 was considered statistically significant. Results are expressed as mean±SE.
Four criteria were used to confirm the AP in rats infused with cerulein at a total dose of 50 μg/kg (10 μg/kg/h s.c. ×5 h): macroscopic and microscopic signs of AP, pancreatic edema leading to the increase in pancreatic weight and the rise in serum lipase activities. All these criteria were fulfilled in all rats treated with cerulein.
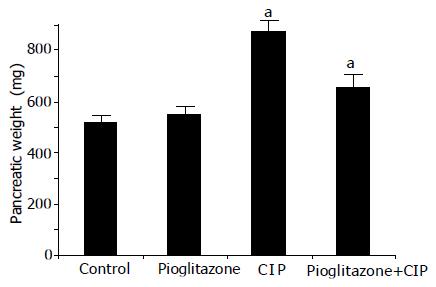
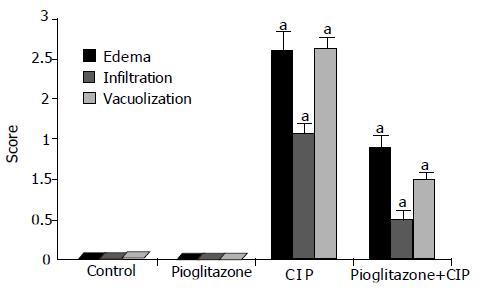
After the infusion of cerulein, the pancreas was enlarged with a visible collection of edematous fluid and the pancreatic mass was almost doubled (Figure 1). At light microscopic level, profound interlobular and intralobular edema, vacuolization of acinar cells, and moderate perivascular and scare diffuse leukocyte infiltration were observed. In rats pretreated with pioglitazone before the start of cerulein infusion, the inflammatory changes in the pancreas were significantly less pronounced when compared to changes observed in rats treated with cerulein alone (Figure 2 and Table 1). Treatment with pioglitazone alone had no effect on morphological features of the pancreas.
| Edema | Leukocyte infiltration | Vacuolization of acinar cells | |
| Control | 0 | 0 | 0 |
| Pioglitazone 10 mg/kg | 0 | 0 | 0 |
| Pioglitazone 20 mg/kg | 0 | 0 | 0 |
| Pioglitazone 50 mg/kg | 0 | 0 | 0 |
| Pioglitazone 100 mg/kg | 0 | 0 | 0 |
| Cerulein | 2/3 | 2 | 2/3 |
| Pioglitazone 10 mg/kg+cerulean | 2/3 | 1/2 | 2 |
| Pioglitazone 20 mg/kg+cerulean | 2 | 1 | 0.5 |
| Pioglitazone 50 mg/kg+cerulean | 1/2 | 0/1 | 1 |
| Pioglitazone 100 mg/kg+cerulean | 1 | 1 | 1 |
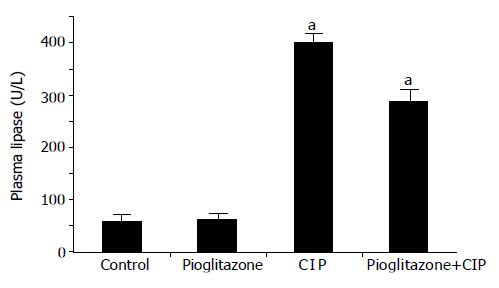
Plasma lipase activity in control, saline-infused rats, reached 57.0±6.0 U/L. In rats infused with cerulein, plasma lipase activity was almost increased eightfold, compared to the level obtained from control rats infused with saline. In rats pretreated with pioglitazone before cerulein, a significant and dose-dependent reduction in plasma lipase activity was found (Figure 3 and Table 2).
| Pancreatic DNA synthesis (dpm/μg DNA) | Pancreatic blood flow (% of control) | Plasma lipase activity (U/L) | Plasma IL-1β (pg/mL) | Plasma IL-10 (pg/mL) | |
| Control | 58.8±1.6 | 100±2.0 | 57.0±6.0 | 70.3±4.2 | 67.0±5.0 |
| Pioglitazone 10 mg/kg | 61.3±1.9 | 98.9±1.7 | 62.3±5.6 | 69.7±3.9 | 66.9±4.1 |
| Pioglitazone 20 mg/kg | 60.9±2.2 | 99.6±2.1 | 59.2±6.7 | 74.2±5.5 | 72.7±5.1 |
| Pioglitazone 50 mg/kg | 61.6±1.1 | 101.5±1.7 | 60.5±4.5 | 68.2±4.9 | 73.0±3.0 |
| Pioglitazone 100 mg/kg | 57.4±2.1 | 97.6±2.4 | 71.8±6.9 | 76.8±5.4 | 71.9±5.1 |
| Cerulein | 33.8±1.4a | 60.9±2.5a | 400.3±10.6a | 212.0±8.6a | 79.2±3.5 |
| Pioglitazone 10 mg/kg+cerulean | 37.6±2.8a | 66.2±2.1a | 372.1±12.1a | 198.4±5.7a | 73.1±3.8 |
| Pioglitazone 20 mg/kg+cerulean | 41.6±1.7a,c | 69.3±1.5a,c | 322.4±9.6a,c | 153.2±6.2a,c | 81.6±7.2 |
| Pioglitazone 50 mg/kg+cerulean | 45.9±1.7a,c | 79.2±2.2a,c | 288.3±18.0a,c | 131.3±7.2a,c | 75.1±3.0 |
| Pioglitazone 100 mg/kg+cerulean | 39.6±2.3a | 65.1±2.3a | 269.3±13.9a | 129.3±6.9a | 64.6±3.2 |
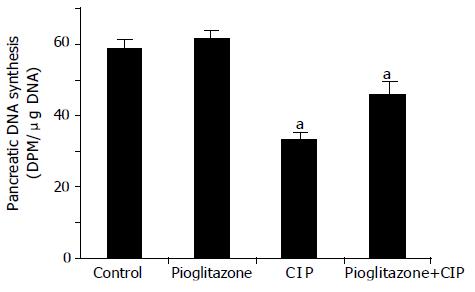
In saline-infused control rats, total pancreatic DNA synthesis reached 58.81.6 dpm/μg DNA (Figure 4). Treatment with pioglitazone alone did not affect pancreatic DNA synthesis in animals infused with saline. The induction of AP by cerulein was associated with a significant fall in DNA synthesis in the pancreas for about 44%. This effect was partly, but significantly reversed by the treatment with pioglitazone.
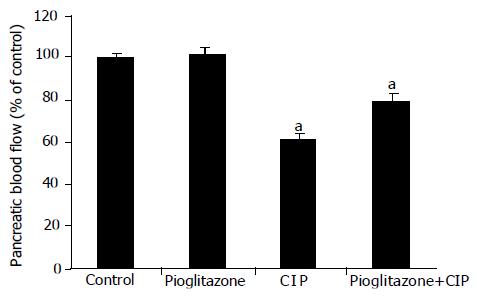
Pancreatic blood flow measured in rats with CIP was reduced by about 40% as compared to the control group (Figure 5). Treatment with pioglitazone did not affect pancreatic blood flow in animals infused with vehicle saline. In contrast, in rats given a cerulein infusion, pretreatment with pioglitazone was associated with a significant reverse in the cerulein-induced fall in the pancreatic blood flow.
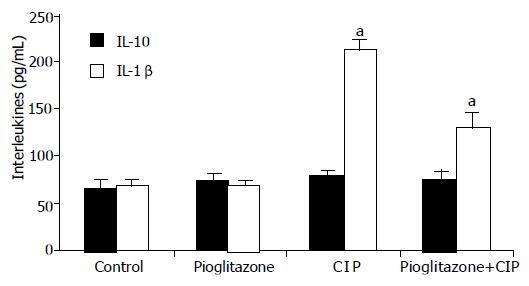
The measurement of plasma level of IL-10 showed no significant changes in rats with CIP or treated with pioglitazone as compared to control group (Figure 6). In contrast, IL-1β was significantly increased in rats with CIP. Pretreatment with pioglitazone did not significantly affect plasma IL-1β concentration in rats infused with saline, but pretreatment with pioglitazone caused a significant and dose-dependent reduction in plasma level of this important pro-inflammatory cytokine in animals with CIP.
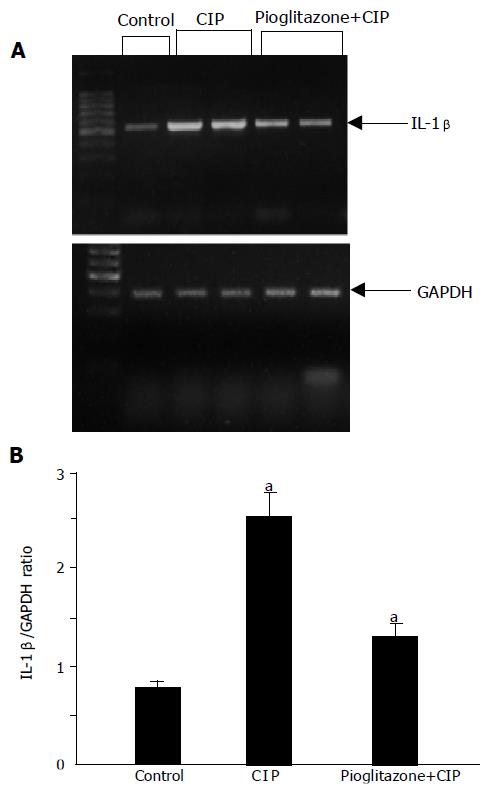
In tissue samples taken from control pancreas without CIP, a weak but detectable signal for IL-β1 mRNA was observed (Figure 7). The expression of GAPDH was well preserved in all the samples. After the induction of CIP, a significant increase in IL-1β mRNA was found. This effect was strongly attenuated in rats pretreated with pioglitazone.
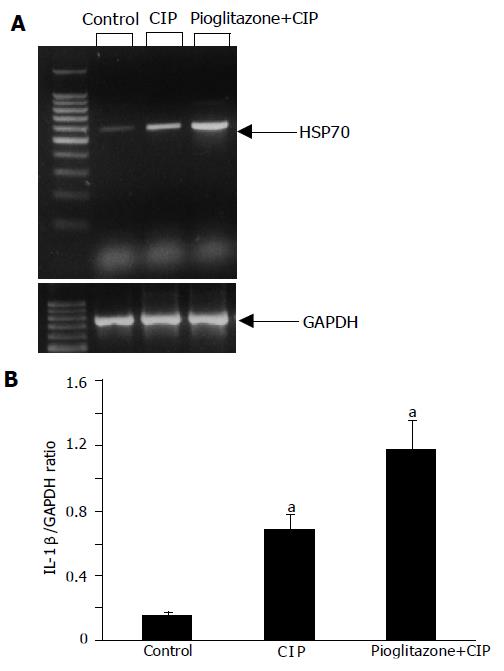
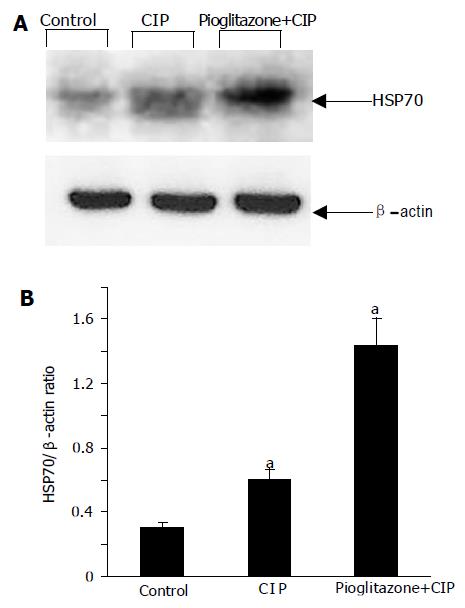
The analysis of mRNA expression of HSP70 showed a detectable signal of HSP70 in the samples of the pancreas obtained from control group (Figure 8). After the induction of CIP, a strong upregulation of mRNA for HSP70 was observed. Interestingly, the treatment with pioglitazone caused a further increase in mRNA expression for HSP70.
At the protein level, similar results were observed; a weak signal for HSP70 in control pancreas, a significant increase of the signal in CIP, and a further increase in HSP70 expression in rats with CIP pretreated with pioglitazone (Figure 9).
We have reported in this study that the pre-treatment of rats with pioglitazone, a specific PPARγ ligand, significantly and dose-dependently attenuated the development of CIP. This result is in accordance with the earlier report that natural PPARγ ligand, 15d-PGJ2, reduced the severity of CIP[19]. The mechanisms underlying the protective effect of pioglitazone on the CIP remain unclear. First of all, the beneficial effect of pioglitazone on the pancreas subjected to acute injury by cerulein over-stimulation could be due to its local and systemic anti-inflammatory properties. Previous reports indicated that PPARγ agonists exhibit anti-inflammatory effects both in in vitro and in vivo studies[5,19]. This anti-inflammatory effect seems to be related to multiple mechanisms. Ricote et al[20], demonstrated that PPARγ ligands inhibit macrophage activation and inflammatory cytokine production. Our previous studies showed that pioglitazone reduces acute and chronic gastric mucosal lesions due to the inhibition of TNF-α and IL-1β expression[12]. Furthermore, Meier et al[21], showed that PPARγ ligands exert anti-inflammatory activity by inducing the production of IL-1 receptor antagonist. One of the important mechanisms by which pioglitazone inhibits the inflammatory reaction is the reduction in activation of the transcription factor, NFκB, by the IκB-dependent mechanism[19]. Moreover, Imamoto et al[22], found that pioglitazone exerts a strong inhibitory effect on the expression of adhesion molecule VCAM-1 on neutrophils and endothelial cells, thus reducing the leukocyte recruitment in the inflamed tissue. There is also clinical evidence for the anti-inflammatory effects of glitazones. Haffner et al[23], reported that treatment of type 2 diabetes mellitus patients with rosiglitazone resulted in a reduction of serum concentration of C-reactive protein and WBCs count. All these reports underline the importance of PPARγ ligands in the modulation of inflammatory reaction in different organs, particularly in reducing the cerulein-induced oxidative stress in pancreatic tissue[24].
Our present study confirms and extends previous findings[25-27] by showing that inflammatory infiltration of pancreatic tissue caused by CIP is accompanied by a significant increase in pancreatic mRNA expression of IL-1β and increased release of this cytokine in rats infused for 5 h with supramaximal dose of cerulein. The new finding of our present study is that pretreatment with pioglitazone at a dose of 50 mg/kg significantly suppresses the gene expression and plasma level of IL-1β, as well as reduces leukocyte infiltration of pancreatic tissue in the course of CIP. This observation indicates that pioglitazone, a specific PPARγ ligand, is a powerful modulator of inflammatory response.
In contrast to IL-1β, IL-10 is found to be a major anti-inflammatory cytokine[28]. It reduces activation of macrophages and inhibits the production of reactive oxygen species and pro-inflammatory cytokines[28]. Van Laethem et al[29], showed that administration of IL-10 before and during the induction of AP decreases the severity of this pancreatitis. Also, administration of some growth factor such as hepatocyte growth factor[30] or insulin-like growth factor-1[13] increases a plasma level of IL-10 and inhibits the development of AP. In our present study, we did not observe any alteration in plasma IL-10 concentration after the development of acute CIP or pretreatment with pioglitazone at various doses. This result suggests that the anti-inflammatory action of pioglitazone does not depend upon the release of IL-10.
The most important finding of this study is the observation that pioglitazone increased significantly the expression of HSP70. HSPs are highly conserved cytoprotective proteins that have essential functions in protein folding, transport, degradation, and assembly. HSPs are classified into six major families (small HSP, HSP40, HSP60, HSP70, HSP90, and HSP100) depending on their molecular weight[7]. HSP70 is the best studied member of HSPs family. Previous studies demonstrated that both thermal and non-thermal stress protects against CIP and prevents trypsinogen activation in the pancreas and this effect is mediated by HSP70[31]. The potential protective mechanisms of HSPs on pancreas include: stabilization and refolding of damaged proteins, resistance of the cells to apoptosis or necrosis, decrease in the level of pro-inflammatory cytokines and antioxidant effects and interference with intra-acinar zymogen activation[7,31-33]. Our results are in accordance with the previous studies showing an increase of HSP70 at mRNA and protein level after induction of CIP. The major finding is, however, the observation of the further increase in HSP70 expression in rats pretreated with pioglitazone. This indicates that the protective effects of pioglitazone could be mediated, at least in part, through increased HSP expression. This issue needs to be clarified in further studies, especially in in vitro studies.
Administration of pioglitazone alone did not affect pancreatic DNA synthesis. Induction of AP by cerulein caused a reduction in the pancreatic DNA synthesis, that is in agreement with our previous observation[34], and may be considered as an index of pancreatic damage. In rats with the induction of pancreatitis by cerulein, the pretreatment with pioglitazone attenuated the pancreatitis-evoked fall in pancreatic DNA synthesis. This observation is an additional evidence of protective effect of pioglitazone against pancreatic damage in CIP.
It is well known that the development of AP is associated with the reduction in pancreatic blood flow[35-37], whereas vasodilatation and the improvement of pancreatic blood flow reduces the development of AP[37]. Our results are in accordance with these observations. Administration of cerulein induced acute edematous pancreatitis and reduced the pancreatic blood flow. Pretreatment with pioglitazone partly reversed cerulein-induced fall in pancreatic blood flow, suggesting that the improvement of pancreatic microcirculation might contribute to the protective effect of pioglitazone administration. The mechanism of vascular effects of pioglitazone administration is unclear. Pioglitazone given alone without cerulein infusion had no effect on pancreatic blood flow. This observation indicates that pioglitazone acts indirectly on pancreatic blood flow, probably by the reduction of cerulein-induced pancreatic edema and reduction of leukocyte activation.
The fact that pioglitazone strongly inhibits the inflammatory reaction in CIP, suggests that this compound may offer a new treatment opportunity, especially in the prophylaxis of pancreatitis in human beings. This issue should be studied in the prospective protocol in human beings. Moreover, the protective effect of pioglitazone should be checked in other acute and chronic experimental models of pancreatitis.
In summary, our study provides evidence that pioglitazone, a specific PPARγ ligand, ameliorates significantly the pancreatic damage associated with the CIP confirmed by the improvement of pancreas histology and the reduction in plasma lipase activity. Treatment with pioglitazone significantly inhibited the production and release of IL-1β and enhanced the expression of pancreatic HSP70. These observations indicate that the treatment with pioglitazone may offer a new therapeutic option in the treatment or prophylaxis of pancreatitis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Kingsnorth A. Role of cytokines and their inhibitors in acute pancreatitis. Gut. 1997;40:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and inflammation: from basic science to clinical applications. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S41-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5272] [Cited by in RCA: 5179] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 4. | Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1865] [Cited by in RCA: 1956] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 5. | Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1372] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 6. | Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 611] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Rakonczay Z, Takács T, Boros I, Lonovics J. Heat shock proteins and the pancreas. J Cell Physiol. 2003;195:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003;304:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Wagner AC, Weber H, Jonas L, Nizze H, Strowski M, Fiedler F, Printz H, Steffen H, Goke B. Hyperthermia induces heat shock protein expression and protection against caerulein-induced pancreatitis in rats. Gastroenterology. 1996;111:1333-1342. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Tashiro M, Ernst SA, Edwards J, Williams JA. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Konturek PC, Jaworek J, Maniatoglou A, Bonior J, Meixner H, Konturek SJ, Hahn EG. Leptin modulates the inflammatory response in acute pancreatitis. Digestion. 2002;65:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Konturek PC, Brzozowski T, Kania T, Konturek SJ, Kwiecien S, Pajdo R, Hahn EG. Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, accelerates gastric ulcer healing in rats. Eur J Pharmacol. 2003;38:468-476. |
| 13. | Konturek SJ, Szlachcic A, Dembinski A, Warzecha Z, Jaworek J, Stachura J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol. 1994;15:19-28. [PubMed] |
| 14. | Warzecha Z, Dembinski A, Ceranowicz P, Konturek SJ, Tomaszewska R, Stachura J, Konturek PC. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J Physiol Pharmacol. 2003;54:575-590. [PubMed] |
| 15. | Warzecha Z, Dembiński A, Konturek PC, Ceranowicz P, Konturek SJ. Epidermal growth factor protects against pancreatic damage in cerulein-induced pancreatitis. Digestion. 1999;60:314-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Giles KW, Myers A. An improvement diphenylamine method for the estimation of deoxyribonucleic acid. Nature. 1965;206:93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1482] [Cited by in RCA: 1350] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 17. | Warzecha Z, Dembiński A, Ceranowicz P, Konturek S, Tomaszewska R, Stachura J, Nakamura T, Konturek PC. Inhibition of cyclooxygenase-2 reduces the protective effect of hepatocyte growth factor in experimental pancreatitis. Eur J Pharmacol. 2004;486:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Konturek PC, Brzozowski T, Kania J, Konturek SJ, Hahn EG. Nitric oxide-releasing aspirin protects gastric mucosa against ethanol damage in rats with functional ablation of sensory nerves. Inflamm Res. 2003;52:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Hashimoto K, Ethridge RT, Saito H, Rajaraman S, Evers BM. The PPARgamma ligand, 15d-PGJ2, attenuates the severity of cerulein-induced acute pancreatitis. Pancreas. 2003;27:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2807] [Cited by in RCA: 2817] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 21. | Meier CA, Chicheportiche R, Juge-Aubry CE, Dreyer MG, Dayer JM. Regulation of the interleukin-1 receptor antagonist in THP-1 cells by ligands of the peroxisome proliferator-activated receptor gamma. Cytokine. 2002;18:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Imamoto E, Yoshida N, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, Naito Y, Tanigawa T, Yoshikawa T. Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 2004;20:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 624] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 24. | Fu K, Sarras MP, De Lisle RC, Andrews GK. Expression of oxidative stress-responsive genes and cytokine genes during caerulein-induced acute pancreatitis. Am J Physiol. 1997;273:G696-G705. [PubMed] |
| 25. | Norman JG, Fink GW, Denham W. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783-1788. [RCA] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Warzecha Z, Dembiński A, Ceranowicz P, Konturek PC, Stachura J, Tomaszewska R, Konturek SJ. Calcitonin gene-related peptide can attenuate or augment pancreatic damage in caerulein-induced pancreatitis in rats. J Physiol Pharmacol. 1999;50:49-62. [PubMed] |
| 27. | Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1743] [Cited by in RCA: 1733] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 28. | de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2625] [Cited by in RCA: 2836] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 29. | Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Warzecha Z, Dembiński A, Konturek PC, Ceranowicz P, Konturek SJ, Tomaszewska R, Schuppan D, Stachura J, Nakamura T. Hepatocyte growth factor attenuates pancreatic damage in caerulein-induced pancreatitis in rats. Eur J Pharmacol. 2001;430:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Frossard JL, Bhagat L, Lee HS, Hietaranta AJ, Singh VP, Song AM, Steer ML, Saluja AK. Both thermal and non-thermal stress protect against caerulein induced pancreatitis and prevent trypsinogen activation in the pancreas. Gut. 2002;50:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22:9041-9047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | Bhagat L, Singh VP, Hietaranta AJ, Agrawal S, Steer ML, Saluja AK. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest. 2000;106:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Dembinski A, Warzecha Z, Konturek PC, Ceranowicz P, Konturek SJ, Tomaszewska R, Stachura J. Adaptation of pancreas to repeated caerulein-induced pancreatitis in rats. J Physiol Pharmacol. 1996;47:455-467. [PubMed] |
| 35. | Menger MD, Plusczyk T, Vollmar B. Microcirculatory derangements in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2001;8:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Zhou ZG, Chen YD, Sun W, Chen Z. Pancreatic microcirculatory impairment in experimental acute pancreatitis in rats. World J Gastroenterol. 2002;8:933-936. [PubMed] |
| 37. | Warzecha Z, Dembiński A, Ceranowicz P, Konturek PC, Stachura J, Konturek SJ, Niemiec J. Protective effect of calcitonin gene-related peptide against caerulein-induced pancreatitis in rats. J Physiol Pharmacol. 1997;48:775-787. [PubMed] |