Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6262
Revised: May 21, 2005
Accepted: May 24, 2005
Published online: October 28, 2005
AIM: To design a classification tool for the histological assessment of hepatocellular carcinoma (HCC), dysplastic nodules (DN), and macroregenerative nodules (MRN) in cirrhotic liver.
METHODS: Two hundred and twelve hepatocellular nodules (106 HCC; 74 MRN; 32 DN) were assessed systematically, quantitatively, and semiquantitatively as appropriate for 10 histological features that have been described as helpful in distinguishing small HCC, DN, and MRN in cirrhotic livers. The data were analyzed by multiple correspondence analysis (MCA).
RESULTS: MCA distributed HCC, DN, and MRN as defined by traditional histological evaluation as well as the individual histological variables, in a “malignancy scale”. Based on the MCA data representation, we created a classification tool, which categorizes an individual nodular lesion as MRN, DN, or HCC based on the balance of all histological features (i.e., vascular invasion, capsular invasion, tumor necrosis, tumor heterogeneity, reticulin loss, capillarization of sinusoids, trabecular thickness, nuclear atypia, and mitotic activity). The classification tool classified most (83%) of a validation set of 47 nodules in the same way as the routine histological assessment. No discrepancies were present for DN and MRN between the routine histological assignment and the classification tool. Of 25 HCC assigned by routine assessment in the validation set, 8 were assigned to the DN category by the classification tool.
CONCLUSION: We have designed a classification tool for the histological assessment of HCC and its putative precursors in cirrhotic liver. Application of this tool systematically records histological features of diagnostic importance in the evaluation of small HCC.
- Citation: Quaglia A, Jutand M, Dhillon A, Godfrey A, Togni R, Bioulac-Sage P, Balabaud C, Winnock M, Dhillon A. Classification tool for the systematic histological assessment of hepatocellular carcinoma, macroregenerative nodules, and dysplastic nodules in cirrhotic liver. World J Gastroenterol 2005; 11(40): 6262-6268
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6262
The current histological classification of hepatocellular carcinoma (HCC) and its putative precursor nodular lesions is controversial and unsatisfactory, particularly with small lesions of 1-2 cm in diameter. Most of the histological criteria for the evaluation of early HCC and its putative precursors have not been validated properly, and the diagnosis of these lesions still depends largely on subjective interpretation of histological features, which are rarely recorded either in routine diagnostic practice or in the published literature.
The aim of this study was to derive a classification tool based on individual histological features that enable the distinction between HCC, dysplastic nodules (DN), and macroregenerative nodules (MRN) in cirrhotic liver. The design of this classification tool was based on the correspondence between the routine histological classification of liver cell nodules and the classification of the same liver cell nodules by a systematic analysis of the individual histological features that may contribute towards the subjective diagnostic assignment. Lesion size was considered specifically in addition because this aspect is currently the most important item that determines the management of HCC in cirrhotic patients according to the recent Barcelona guidelines[1].
Two hundred and twelve liver hepatocellular nodules were retrieved from the files of the Department of Histopathology of the Royal Free Hospital using the Liver Tumor Database of the Royal Free Liver Pathology Unit. These nodules had been isolated during the routine diagnostic pathological examination of the cirrhotic livers, which were removed from 321 consecutive liver transplant patients who received liver transplantation at the Royal Free Hospital between 1996 and 2001 for various etiologies. Sixty-eight of these patients (59 males, 26 HCV, 19 HBV, 10 cryptogenic cirrhosis, 6 alcoholic liver disease, 3 alcoholic liver disease and HCV, 2 HCV and HBV, 1 Wilson’s disease, 1 primary biliary cirrhosis) were found to have HCC in the explanted liver, and/or DN and/or MRN, and livers of these patients constitute our study group. For each nodule, a hematoxylin and eosin (H&E)-stained section was performed as part of the routine histological assessment, as well as a silver impregnation for reticulin fibers using the Gordon and Sweets’ method and an immunohistochemical staining for CD34 and smooth muscle actin (SMA).
The overall assessment was performed assigning each liver cell nodule to one of the three groups: HCC; DN; or MRN by “traditional” subjective diagnostic assessment (APD), using the histological criteria defined by Ferrell et al[2], and the International Working Party[3]. The systematic scoring procedure (AQ) was conducted separately from the overall assignment, in order to eliminate bias.
The following histological features were chosen for the systematic histological assessment of each lesion: nodule size, nodule heterogeneity, reticulin loss, trabecular thickness, capillarization, number of solitary arterioles, cellular atypia and mitotic activity, necrosis, vascular invasion, and capsular invasion.
These features were chosen because they are currently considered by many liver pathologists as the most useful histological criteria in the histological assessment of hepatocellular lesions, as it appears from published work on this subject[4-8]. Each lesion was reviewed with systematic documentation of these individual features, and a semiquantitative score was given to reticulin loss, capillarization, and cellular atypia (Table 1), as described below.
| Feature | Modality of assessment | |||||
| Nodule maximum diameter | Measurement in millimeter | |||||
| Solitary arterioles | Average of individual arteries ×100 (medium power field, MPF) fields | |||||
| Mitotic activity | Number of mitoses per 10 ×400 (10 high power fields, 10 HPF) fields | |||||
| Trabecular thickness | Number of liver cells forming trabecular (LCT) width | |||||
| Vascular invasion | Present/absent | |||||
| Capsular invasion | Present/absent | |||||
| Necrosis2 | Present/absent | |||||
| Nodule heterogeneity1 | Present/absent | |||||
| Reticulin loss3 | None | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Capillarization (CD34)3 | None | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Cellular atypia3 | None | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
Size The evolution from cirrhotic liver to MRN, DN, and HCC is usually accompanied by an increase in nodule size. In other words, the greater the size of the lesion, the greater the likelihood that it is a dysplastic nodule or HCC[2-4,7,9,10]. Size was determined macroscopically as maximum diameter of the lesion and expressed in millimeters.
Reticulin loss This has been traditionally considered to be a diagnostic feature of HCC. The following scale was used: grade 0 was given when the reticulin stroma was preserved; grade 1-5 was defined by the number of liver cells included between residual strands of silver-staining reticulin, with grade 1 when liver plates were 3-cell thick; and subsequent 1-cell increments up to grade 5 when liver plates were 7-cell thick or more.
Trabecular thickness This means, the number of liver cells composing a hepatocyte plate and may be considered as a corollary of reticulin loss (see above). In overt HCC, hepatocyte plates tend to be thicker than normal[3,11]. Trabecular thickness was assessed using H&E staining and graded as number of liver cells composing a trabecular plate.
Arterialization and capillarization Number of solitary arterioles[12-14] and capillarization[15] are related to the changes of vascular supply in the evolution of HCC from precursor nodular lesions including acquisition of a predominantly arterial vascular supply and of a diffuse pattern of expression of CD34 by sinusoidal endothelium.
Capillarization was graded as 0 when marginal (i.e., staining only septal endothelium and endothelium of limiting plate of the nodule); Patchy (grades 1-2) when non-confluent patches (grade 1 = in one-third of the nodule; grade 2 in at least two-thirds of the nodule) showing diffuse CD34 immunostaining were seen; incomplete (grade 3) when confluent areas showing diffuse CD34 immunostaining were seen, occupying approximately two-thirds of the nodule; diffuse incomplete (grade 4) when diffuse CD34 staining was seen in the whole nodule apart from scattered CD34-ve patches; diffuse (grade 5) when the entire nodule stained for CD34.
Solitary arterioles: these were counted as average of unpaired arteries detected in 10 medium (100×) power fields, on H&E sections[14].
Cellular atypia and mitotic activity The International Working Party included both cellular atypia and mitotic activity in morphological diagnostic criteria of HCC[3]. Cellular atypia was graded as mild (grades 1-2), moderate or severe (grades 4-5) depending on the similarity in terms of features, such as nuclear contour, hyperchromatism, and nuclear cytoplasmic ratio, when compared to background cirrhotic liver[8] with mild atypia described as minimal difference (and grade 1 or 2 depending on the extent of the changes within the nodule, grade 1 in 1/3 of the surface examined; grade 2 in 2-3/3 of the surface examined), severe atypia as prominent nuclear changes with marked pleomorphism and severe hyperchromatism (and grade 4 or 5 depending on the extent of the changes within the nodule, grade 1 in 1/3 of the surface examined; grade 2 in 2-3/3 of the surface examined), and moderate atypia as intermediate changes.
Mitotic activity was graded as shown in Table 1, counting the number of mitoses in 10 high (400×) power fields[3].
Heterogeneity This term describes the morphological changes seen in a hepatocellular lesion and presumably related to the clonal evolution seen in the development of HCC. This is evident morphologically, for example, as a “nodule in nodule pattern” and consists of areas with one or more morphological changes distinct from the parent nodule and often with compression of the parent nodule suggesting an increased growth rate. These morphological changes include small cell change, microacinar change, clear cell change, fatty change, groups of Mallory body or fibrinogen containing cells, and loss or accumulation of iron or bile compared to the background liver[3,4].
Tumor necrosis usually occurs when cell growth exceeds the vascular supply and is usually seen in tumors at a relatively advanced biological stage. Necrosis was considered to be diagnostically relevant only when it was “spontaneous” in the absence of previous history of pre-transplantation local treatment (e.g., trans-arterial chemoembolization, percutaneous ethanol injection, etc.).
Invasion Vascular invasion was considered as invasion of vascular structures identified histologically. Capsular invasion was recorded when there was invasion of perinodular dense fibrous tissue[5,8,16].
For each nodule, H&E-stained section was performed as part of the routine histological assessment, as well as a silver impregnation for reticulin fibers using the Gordon and Sweets’ method and an immunohistochemical staining for CD34 and SMA.
The classification tool (Table 3) was tested prospectively on a subsequent separate set of hepatocellular nodules identified in 11 consecutive liver explants with 47 distinct nodular liver cell lesions removed at transplantation in the year 2002. The test set of nodules consisted of 25 HCC (mean size, 19.4 mm; SD, 14 mm), 9 DN (mean size, 10.7 mm; SD, 3 mm), and 13 MRN (mean size, 7.7 mm; SD, 2.2 mm).
| Column 1 | Column 2 | Column 3 | Column 4 |
| HCC | DN or MRN | DN | MRN |
| Vascular invasion not present | Capillarization score 3, 4, or 5 | Capillarization score 0, 1, or 2 | |
| Capsular invasion not present | Cellular atypia score 1, 2, 3, 4, or 5 | Cellular atypia score 0 | |
| Necrosis present | Necrosis not present | Reticulin loss score 1, 2, 3, 4, or 5 | Reticulin loss score 0 |
| Mitosis present | Mitosis not present | Trabecular thickness >3 | Trabecular thickness <4 |
| Capillarization score 4 or 5 | Capillarization Score 0, 1, 2 or 3 | Solitary arterioles ≥1 | Solitary arterioles <1 |
| Cellular atypia score 3, 4, or 5 | Cellular atypia score 0, 1, or 2 | Heterogeneity present | Heterogeneity absent |
| Reticulin loss score 1, 2, 3, 4, or 5 | Reticulin loss score 0 | Necrosis present | |
| Trabecular thickness>3 | Trabecular thickness<4 | Mitosis present | |
| Solitary arterioles ≥1 | Solitary arterioles<1 | ||
| Heterogeneity present | Heterogeneity absent |
The individual histological features described above were analyzed by multiple correspondence analysis (MCA). MCA is an exploratory technique[17] allowing the synthesis, description, and graphic analysis of large contingency tables. The results provide information that is similar in nature to those produced by factor analysis techniques, and they allow one to explore the structure of categorical variables included in the table. No tests of statistical significance are applied to the results and the main goal is to produce a “simplified” representation of the data.
In the present work, MCA was used in two ways: (1) To see whether this method could reliably categorize HCC, DN, and MRN using the individual histological features described above, and how this analysis compared to the overall (routine) histological assessment. MCA produces a graphic representation of the data in the form of a horizontal line, which represents a “malignancy” scale and shows how each nodule is placed in a “malignancy” scale. In theory, malignant nodules are placed at one end of the scale and benign nodules are placed at the opposite end of the scale, with equivocal nodules in between. Each variable has a certain value in an axis, and the 《position》 in the chart represents the composite point of different planes (features) for a given nodule; (2) To see if this method could rank the individual histological features in order to assess the relative contribution of each individual histological observation in the assignment of a liver cell nodule to one of the three categories (HCC, DN, and MRN), so that the histological features could be incorporated into a classification tool to distinguish HCC from DN from MRN.
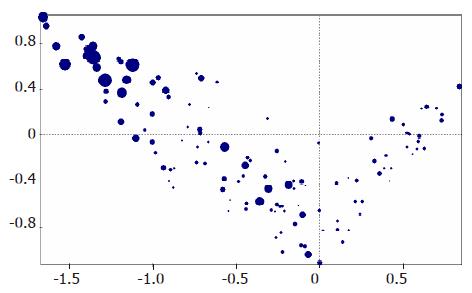
An exploratory set of 212 hepatocellular nodules (106 HCC mean size 18.8 mm, SD 13 mm; 74 MRN mean size 10 mm, SD 3.2 mm; 32 DN mean size 12.4 mm, SD 5.5 mm, as per routine histological assessment) were analyzed histologically. Figure 2 shows how MCA redistributes these nodules. The horizontal line represents a “malignancy” scale. In other words, the projection of every dot towards the horizontal line (e.g., three dotted lines in chart) shows how each nodule is placed in a “malignancy” scale. HCC are placed in the left-hand side of the chart, whereas MRN are placed towards the other end. Each variable has a certain value in an axis, and the « position » in the chart represents the resulting point of different planes for a given nodule. Size (Figure 1) does not allow a reliable classification of nodules. In other words, HCC can be of all sizes, and although large nodules are usually HCC, small nodules may also be HCC. Conversely, MRN and DN can be of considerable size (up to 21 and 24 mm, respectively, in our series).
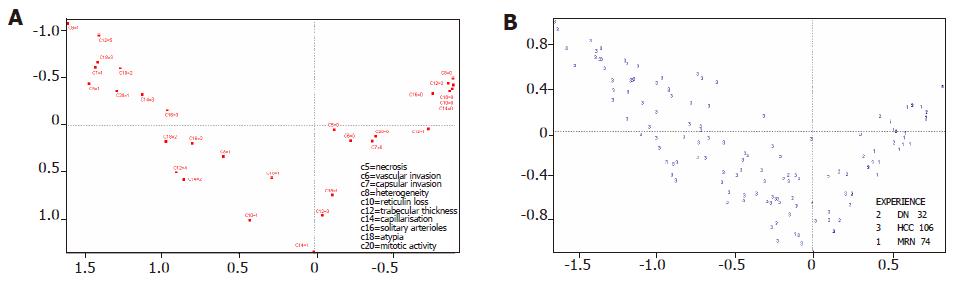
Figure 2B shows how the individual variables (e.g., capsular invasion, vascular invasion, etc.) relate to each other, and how they are distributed along the “malignancy scale”. As in Figure 2A, the horizontal line represents a “scale of malignancy”. The projection of each group of values in the horizontal axis shows that the variables form a continuum, with capsular invasion being the “most” malignant feature, and absence of heterogeneity the “least” malignant feature. Table 2 shows how the groups of values depicted in Figure 2B are distributed in the three types of nodular lesions.
| Position | Feature | Grade | HCC number (total 106) | % | DN number (total 32) | % | MRN number (total 74) | % |
| 1 | Vascular invasion | Present | 27 | 25.5 | 0 | 0 | 0 | 0 |
| 2 | Necrosis | Present | 17 | 16 | 0 | 0 | 0 | 0 |
| 3 | Capsular invasion | Present | 44 | 41.5 | 0 | 0 | 0 | 0 |
| 4 | Cellular atypia | 4-5 | 30 | 28.3 | 0 | 0 | 0 | 0 |
| 5 | Trabecular thickness | >4 | 35 | 33 | 1 | 3 | 0 | 0 |
| 6 | Mitosis | Any | 50 | 47.2 | 0 | 0 | 0 | 0 |
| 7 | Reticulin loss | 4-5 | 47 | 44.3 | 0 | 0 | 0 | 0 |
| 8 | Capillarization | 5 | 56 | 52.8 | 0 | 0 | 0 | 0 |
| 9 | Cellular atypia | 3 | 38 | 35.8 | 0 | 0 | 0 | 0 |
| 10 | Solitary arterioles | >2 | 23 | 21.7 | 1 | 3 | 1 | 1 |
| 11 | Trabecular thickness | 4 | 27 | 25.5 | 3 | 9 | 0 | 0 |
| 12 | Capillarization | 4 | 27 | 25.5 | 1 | 3 | 0 | 0 |
| 13 | Solitary arterioles | 2 | 37 | 34.9 | 5 | 15 | 1 | 1 |
| 14 | Heterogeneity | Present | 100 | 94.3 | 23 | 72 | 5 | 7 |
| 15 | Reticulin loss | 1-3 | 56 | 52.8 | 9 | 28 | 2 | 3 |
| 16 | Solitary arterioles | 1 | 33 | 31.1 | 7 | 22 | 10 | 14 |
| 17 | Capillarization | 3 | 19 | 17.9 | 8 | 25 | 2 | 3 |
| 18 | Trabecular thickness | <4 | 44 | 41.5 | 27 | 84 | 72 | 97 |
| 19 | Cellular atypia | 1-2 | 37 | 34.9 | 12 | 38 | 16 | 22 |
| 20 | Necrosis | Not present | 89 | 84 | 32 | 100 | 74 | 100 |
| 21 | Vascular invasion | Not present | 79 | 74.5 | 32 | 100 | 74 | 100 |
| 22 | Capsular invasion | Not present | 62 | 58.5 | 32 | 100 | 74 | 100 |
| 23 | Mitosis | Not present | 56 | 52.8 | 32 | 100 | 74 | 100 |
| 24 | Solitary arterioles | <1 | 13 | 12.3 | 19 | 59 | 62 | 84 |
| 25 | Capillarization | 0–2 | 4 | 3.8 | 23 | 72 | 72 | 97 |
| 26 | Cellular atypia | 0 | 1 | 0.9 | 20 | 63 | 58 | 78 |
| 27 | Heterogeneity | Not present | 6 | 5.7 | 9 | 28 | 69 | 93 |
| 28 | Reticulin loss | 0 | 3 | 2.8 | 23 | 72 | 72 | 97 |
These data were used to create a classification tool for the assessment of these nodular lesions.
The values shown in Figure 2 and Table 2 can be distributed into six main groups, depending on their proportional representation in the three categories HCC, DN, and MRN, as follows:
Group 1 (capsular invasion, vascular invasion). Features present exclusively in HCC 44 (41.5%) and 27 (25.5%), respectively, by definition.
Group 2 (necrosis present, mitosis present, capillarization = 5, cellular atypia = 3-5, reticulin loss = 4-5, trabecular thickness>4). Features present, in 16-52.8% (mean 36%) of HCC but not present in MRN or DN.
Group 3 (solitary arterioles ≥1, heterogeneity present, capillarization = 4, reticulin loss = 1-3, trabecular thickness = 4). Features present in 21.7-94.3 (mean = 47%) HCC, 3-72% (mean = 37%) DN and 0-14% (mean = 20%) MRN.
Group 4 (capsular invasion not present, vascular invasion not present, necrosis not present, mitosis not present). Features present in 100% MRN and DN, and in 53-84% (mean 67%) of HCC.
Group 5 (capillarization = 3, cellular atypia = 1-2). Features present in 25-38% (mean = 31%) DN, 17.9-34.9% (mean = 26%) HCC and 3-22% (mean = 12%) MRN.
Group 6 (solitary arterioles<1, heterogeneity not present, capillarization = 0-2, reticulin loss = 0, trabecular thickness<4). Features present in 78-97% (mean = 91%) MRN, 59-72% (mean = 63%) DN and 0.9-41.5 (mean = 11%) HCC.
These six groups can be organized into a three column table as shown in Table 3. This table constitutes the classification tool.
The histological features of a given nodule are assessed individually and then allocated to the classification tool.
This classification tool, when tested prospectively on the separate set of liver cell nodules described above gave the following results: out of 47 nodules, 39 (83%) were classified in the same way as the routine histological assessment. All 13 MRN and all 9 DN, as defined by routine histological assessment were considered as such by the classification tool. Of the 25 HCC, as defined by routine histological assessment, 8 were considered as DN by the classification tool (Table 4).
| Histological assessment usingthe classification tool | ||||||
| HCC | DN | MRN | Total | |||
| Routine | HCC | 25 | 17 | 8 | 0 | 25 |
| Histologica | lDN | 9 | 0 | 9 | 0 | 9 |
| Diagnosis | MRN | 13 | 0 | 0 | 13 | 13 |
| Total | 17 | 17 | 13 | Total | ||
Despite the consensus document published by the International Working Party[3], the terminology used in the literature is still confusing, particularly due to the use of different classifications and different histological criteria by different centers[6]. In fact, the bulk of the relevant literature offers few clues about which precise observations allocated any particular lesion (however, named) into the given categorical assignment. In this work, we have investigated how those individual histological features considered in the literature will be helpful in the histological assessment of small hepatocellular nodular lesions in cirrhosis that can be used to create a classification tool for the evaluation of these nodules. Application of the classification tool documents systematically the individual histological features that contribute to the categorical assignment of MRN, DN, and HCC.
MCA is a descriptive technique, which identifies the best factor representing a set of data. In the present analysis, the horizontal factor, which could be called “malignancy” is the most important one in representing the dataset. The nodular lesions of the present study can be imagined as a “cluster” of dots in space, and the distance between two points depends on the plane (axis of assessment, or histological features) used to look at them. For example, if the horizontal axis is used to look at two lesions, and the projections of these lesions are close together on that axis, these lesions may be considered to be at a similar degree of malignancy. The same lesions on another plane may be very far apart from each other.
MCA distributes the 212 lesions assessed by the components of the histological assessment placing HCC at the left and MRN at the right of the “malignancy” scale, and DN in the middle, although some “misclassifications” are present, with overlap between different categories (Figure 2A), i.e. between HCC and DN and DN and MRN. This is not unexpected, as this analysis represents the nodular lesions as a continuum in a “malignancy” scale. Moreover (Figure 2A), one could also imagine different degrees of malignancy within the HCC subgroup.
When tested on a second set of nodules, the classification tool classified the large majority of all nodules (83%) in the same way as the routine histological assessment. No discrepancies were noted for DN and MRN between the routine diagnosis and the assignment by the classification tool. Eight of twenty-five HCC (32% of HCC, 17% of all nodules, as defined by routine histological assessment) were assigned to the DN category by the classification tool, which is not surprising given the well-recognized difficulties in separating these two categories[3]. The reason for this discrepancy is obscure, and underlines the need for correlation with other modalities of investigation, such as molecular data and clinical follow-up, which may be informative to validate the histological criteria[3] (and this will be the subject of further work). Overall, the classification tool seems to correspond reasonably well to the routine histological assessment.
The classification tool has several advantages. Firstly, the idea of including all important histological features in the evaluation, systematically ensures that the final assignment does not rest on a single feature subjectively or selected group of features, but on the balance of all components. This classification tool is based on a systematic collection of histological data and can be integrated in a database. Once all the fields required for the completion of the classification tool have been entered, a computer can easily run the classification tool and return the result. As more data become available[18], and as additional informative histological features of HCC (such as stromal invasion) are characterized[16,19,20], the accumulated information can be used to improve and refine the classification tool.
In conclusion, we have designed a classification tool for the histological assessment of HCC and its putative precursor nodular lesions in cirrhotic liver. The classification tool is based on a systematic and balanced assessment of 10 histological features.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3244] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 2. | Ferrell LD, Crawford JM, Dhillon AP, Scheuer PJ, Nakanuma Y. Proposal for standardized criteria for the diagnosis of benign, borderline, and malignant hepatocellular lesions arising in chronic advanced liver disease. Am J Surg Pathol. 1993;17:1113-1123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Anonymous, International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 686] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 4. | Theise ND. Macroregenerative (dysplastic) nodules and hepatocarcinogenesis: theoretical and clinical considerations. Semin Liver Dis. 1995;15:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Quaglia A, Bhattacharjya S, Dhillon AP. Limitations of the histopathological diagnosis and prognostic assessment of hepatocellular carcinoma. Histopathology. 2001;38:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kojiro M. Premalignant lesions of hepatocellular carcinoma: pathologic viewpoint. J Hepatobiliary Pancreat Surg. 2000;7:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Bhattacharya S, Davidson B, Dhillon AP. Blood supply of early hepatocellular carcinoma. Semin Liver Dis. 1995;15:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Lauwers GY, Terris B, Balis UJ, Batts KP, Regimbeau JM, Chang Y, Graeme-Cook F, Yamabe H, Ikai I, Cleary KR. International Cooperative Study Group on Hepatocellular carcinoma. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am J Surg Pathol. 2002;26:25-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Furuya K, Nakamura M, Yamamoto Y, Togei K, Otsuka H. Macroregenerative nodule of the liver. A clinicopathologic study of 345 autopsy cases of chronic liver disease. Cancer. 1988;61:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Kondo F, Ebara M, Sugiura N, Wada K, Kita K, Hirooka N, Nagato Y, Kondo Y, Ohto M, Okuda K. Histological features and clinical course of large regenerative nodules: evaluation of their precancerous potentiality. Hepatology. 1990;12:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Scheuer P, Lefkowitch J. Liver Biopsy Interpretation. 6th ed. London, Edimburgh, New York, Philadelphia, St Louis, Sidney, Toronto: W.B. Saunders 2000; 191-227. |
| 12. | Himeno H, Enzan H, Saibara T, Onishi S, Yamamoto Y. Hitherto unrecognized arterioles within hepatocellular carcinoma. J Pathol. 1994;174:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Nakashima Y, Nakashima O, Hsia CC, Kojiro M, Tabor E. Vascularization of small hepatocellular carcinomas: correlation with differentiation. Liver. 1999;19:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol. 2000;33:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 175] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Dhillon AP, Colombari R, Savage K, Scheuer PJ. An immunohistochemical study of the blood vessels within primary hepatocellular tumours. Liver. 1992;12:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Kondo F, Kondo Y, Nagato Y, Tomizawa M, Wada K. Interstitial tumour cell invasion in small hepatocellular carcinoma. Evaluation in microscopic and low magnification views. J Gastroenterol Hepatol. 1994;9:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Lebart L, Morineau A, Piron M. Statistique exploratoire multidimensionnelle. Analyse en composantes principales. 2nd ed. Paris, France: Dunod 1997; 32-66. |
| 18. | Selaru FM, Xu Y, Yin J, Zou T, Liu TC, Mori Y, Abraham JM, Sato F, Wang S, Twigg C. Artificial neural networks distinguish among subtypes of neoplastic colorectal lesions. Gastroenterology. 2002;122:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Miyao Y, Ozaki D, Nagao T, Kondo Y. Interstitial invasion of well-differentiated hepatocellular carcinoma and subsequent tumor growth. Pathol Int. 1999;49:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Tomizawa M, Kondo F, Kondo Y. Growth patterns and interstitial invasion of small hepatocellular carcinoma. Pathol Int. 1995;45:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |