Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.584
Revised: April 6, 2004
Accepted: June 26, 2004
Published online: January 28, 2005
AIM: Helicobacter pylori (H pylori) resistance after failed eradication has a major impact on the outcome of a further treatment regimen. The aim of this study was to assess the validity of a non-invasive strategy using the 13C-urea breath test (UBT) and the gastric string test in identifying post-treatment resistance of H pylori.
METHODS: The UBT was routinely performed 4 to 6 wk after H pylori eradication therapy. Forty-two patients (24 females, 18 males, mean age 48 years) with a positive UBT were included in the study. A gastric string test using a capsule containing a 90 cm-long nylon fiber was performed. Before the capsule was swallowed, the free end of the string was taped to the cheek. After one hour in the stomach, the string was withdrawn. The distal 20 cm of the string was inoculated onto an agar plate and processed under micro-aerophilic conditions. Following the string test, upper gastrointestinal endoscopy was performed to obtain gastric biopsies for conventional culture.
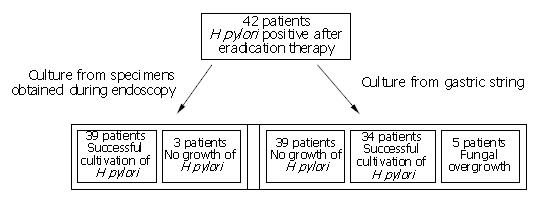
RESULTS: H pylori was successfully cultured from the gastric string in 34 patients (81%), but not in 5 patients due to contamination with oropharyngeal flora. H pylori was cultured from the gastric biopsies obtained at endoscopy in 39 patients (93%).
CONCLUSION: The UBT followed by the gastric string test in the case of treatment failure is a valid diagnostic strategy with the aim of determining the post-therapeutic antibiotic resistance of H pylori with little inconvenience to the patient. Upper GI-endoscopy can be avoided in several cases by applying consequently this diagnostic package.
-
Citation: Leodolter A, Wolle K, von Arnim U, Kahl S, Treiber G, Ebert MP, Peitz U, Malfertheiner P. Breath and string test: A diagnostic package for the identification of treatment failure and antibiotic resistance of
Helicobacter pylori without the necessity of upper gastrointestinal endoscopy. World J Gastroenterol 2005; 11(4): 584-586 - URL: https://www.wjgnet.com/1007-9327/full/v11/i4/584.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.584
The antibiotic resistance of Helicobacter pylori (H pylori) after failed eradication therapy has a major impact on the selection of antibiotics applied in a further treatment regimen[1]. Recent guidelines for the management of H pylori infection recommend triple therapy comprising a proton pump inhibitor (PPI) or ranitidine bismuth citrate, in combination with clarithromycin and amoxicillin or metronidazole, as the first-line therapy, and quadruple therapy (PPI, bismuth, metronidazole and tetracyline) as the second-line therapy in the event of primary treatment failure. The results of a recent meta-analysis revealed failure of primary therapy in about 20-30% of cases[2], and of second line therapy in a further 20-30% of cases[3]. Thus the bacterium persisted after two courses of H pylori eradication therapy in about 10 out of 100 infected patients. In this situation antibiotic susceptibility testing is recommended[4], and treatment of H pylori infection should be based on a susceptibility-adapted therapy regimen. While resistance to amoxicillin is infrequent, resistance to clarithromycin and metronidazole is common after failed H pylori eradication therapy[1], and this implies a markedly decreased eradication rate[5,6].
The post-treatment evaluation of the H pylori status has become a domain of non-invasive testing with either the 13C-urea breath test (UBT) or with the stool antigen test if UBT is not available[4]. For antibiotic susceptibility testing, upper gastrointestinal (GI)-endoscopy with gastric biopsies is the standard procedure. The gastric specimens thus obtained are further processed by microbiologic methods for bacterial culture and the determination of antibiotic susceptibility.
As a possible alternative to upper GI-endoscopy, Perez-Trallero et al[7] proposed a gastric string test. This test enables gastric mucus to be obtained without the need for upper GI-endoscopy. H pylori is readily isolated from the mucus, and this approach has been successfully used to culture H pylori for susceptibility testing in a number of studies[7-10]. Limitations of the gastric string test are the low density of H pylori in the mucus, and contamination with flora from the oropharynx. To overcome these shortcomings we have further modified the test with the aim of introducing a diagnostic package combining the UBT and the gastric string test. The present study was conducted to assess the validity of this diagnostic package in clinical practice for deciding the appropriate management of H pylori resistance.
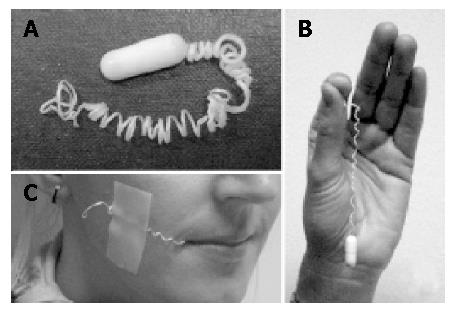
Patients were assigned by GP’s or our Out-Patient Department to receive a UBT 4 to 6 wk after H pylori eradication therapy. A validated test protocol for the UBT was used. After an overnight fast, 200 mL of tap water containing 4.2 g citric acid, 40 mg saccharin and 75 mg 13C-urea was administered to the patients. Breath samples were collected before and 30 min after administration of the test drink. Breath samples were analyzed by an isotope ratio mass spectrometer (ABCA, PDZ Europe Crewe, UK), and the results were expressed as “delta over baseline” (DOB). A DOB >4‰ was considered positive for H pylori[11]. If the UBT was positive and the further inclusion criteria were met (no antibiotics, bismuth-containing compounds, H2-receptor antagonists or proton pump inhibitors during the four weeks preceding the 13C-UBT), the patients underwent a gastric string test. For this test patients swallowed a gelatine capsule containing a 90 cm long string of absorbent fiber (Entero-Test HDC Corporation, San Jose, US) after an overnight fast (Figure 1). Before the capsule was swallowed, the patient was requested to gargle for 30 s with a hexitidine solution. The free end of the string was taped to the cheek. One hour later the patient gargled once more with hexitidine solution and the string was withdrawn. The distal 20 cm of the string was immediately inoculated onto a special agar plate for cultivation of H pylori (Pylori Agar, bioMérieux, France), and samples were incubated for 2 to 10 d in a micro-aerophilic chamber at 37°C. H pylori was identified on the basis of typical morphology using Gram staining, and positive urease, oxidase, and catalase tests. Minimal inhibition concentrations (MIC) were determined using the Etest (AB Biodisk, Sweden) on blood agar plates. Suspensions of H pylori were adjusted to a McFarland standard no. 2 as the inoculum. After a 3-d incubation period under micro-aerophilic conditions, the MICs of clarithromycin and metronidazole were determined. Strains with an MIC >8 mg/L were considered resistant to metronidazole and those with an MIC >1 mg/L were considered resistant to clarithromycin. Following the string test, upper GI-endoscopy was performed in all patients to obtain gastric biopsies (4 biopsies form the antrum and 4 from the corpus for histology, rapid urease test and culture). The biopsies for culture (one from the antrum and one from the corpus) were processed in the same way as the distal part of the string. The study protocol was approved by the Ethics Committee of the University of Magdeburg, and all patients gave their written informed consent.
Forty-two patients (24 females, 18 males, mean age 48 years) out of 50 consecutive patients with a positive UBT consented to undergo the gastric string test. H pylori was cultured from the gastric biopsies in 39 of the 42 patients (93%) and from the gastric string in 34 patients (81%). In one patient H pylori was grown from the gastric string, but not from the biopsies. Fungal contamination prevented cultivation of H pylori in 5 patients, and no growth of H pylori was observed in a further three patients after 10 d of incubation (Figure 2). Resistance to clarithromycin was observed in 24 out of the 40 patients (60%), and to metronidazole in 34 patients (85%). The resistance rate was identical with both investigational methods.
Implementation of the gastric string test was successful in all patients. With one exception, all patients preferred the gastric string test to upper GI-endoscopy. The main reason given by the patient (a 54-year-old man) who favored endoscopy, was the duration of the investigation (about 70 min for the string test).
Our results showed that a minimum of 45 upper GI-endoscopies had to be performed to establish the antibiotic resistance of H pylori. The figure of 45 was the sum of 42 initial endoscopies and 3 additional endoscopies in the patients with no growth of H pylori from biopsies obtained at the initial endoscopy, assuming that H pylori would be grown from all the biopsies taken at the second endoscopy. In contrast, the gastric string test was successful in 34 patients. In the remaining 8 patients two options were possible, namely the gastric string test could be repeated or an upper GI-endoscopy could be performed to obtain biopsies for susceptibility testing. If the latter was the preferred strategy, upper GI-endoscopy would be necessary in only 8 patients to enable H pylori susceptibility to be determined in all the 42 patients, again assuming that H pylori would be grown from all biopsies of the second endoscopy. Overall this implied a maximum reduction in upper GI-endoscopies of about 80%.
The combination of the UBT to determine the H pylori status after eradication therapy, and the minimally invasive gastric string test, in cases of persistent H pylori infection to determine the post treatment antibiotic susceptibility, would appear to be a useful diagnostic package for the management of H pylori infection. Using the gastric string test it was possible to obtain data on the susceptibility of H pylori to clarithromycin and metronidazole in 81% of patients. This package approach is therefore a valid alternative to upper GI-endoscopy with biopsies for susceptibility testing.
The validity of the UBT for pre- and post-treatment diagnoses of H pylori infection has repeatedly been demonstrated[11] and the UBT is generally recommended[4]. In a second step we added the gastric string test to recover bacteria from the individual patients to determine their susceptibility to the two most commonly used antibiotic agents (clarithromycin and metronidazole). However, all other antibiotics of interest in H pylori therapy could also be investigated. Resistance of H pylori to clarithromycin and metronidazole is the principal cause of treatment failure. Resistance to clarithromycin could reduce the therapeutic efficacy by an average of 55.4%, and resistance to metronidazole by an average of 37.7%[12]. Pre-treatment determination of the susceptibility of H pylori has been shown to be cost-effective in an American model using upper GI-endoscopy to obtain the bacteria[13]. Obviously, if the cost of endoscopy could be avoided, cost-effectiveness would be improved. A detailed calculation of savings needs to be performed in the individual community, since impacting factors are multiple: prevalence of resistance to antibiotics, cure rate for sensitive strains, cure rate for resistant strains, and the cost of diagnosing and treating H pylori.
The cultivation rate of H pylori in the gastric string test was lower than that in gastric biopsy materials. However, susceptibility of H pylori to different antibiotics could be identified in about 80% of the patients. In light of these results, about 80% of upper GI-endoscopies may be dispensable. This would be a major advantage for both the patients and the community, since the gastric string test is much cheaper than upper GI endoscopy. The cost of the string capsule is about 25 US$, and the cost of culturing H pylori is identical for both options. On the basis of these calculations, and given the limited resources of endoscopy for obtaining biopsies for H pylori culture, the combination of the UBT and the gastric string test offers an attractive alternative.
However, in our study, this concept presupposed the availability of a microbiologic laboratory in the vicinity. This is not a basic requirement, the string could be transported over up to 24 h at below 50°F /10°C before it was plated on an cultivation agar[14]. The breath sample of the UBT could also be easily transported over long distances in small glass tubes. Therefore the string test as well as the UBT are also applicable far away from a specialized laboratory. Both tests can be easily performed by a medical assistant technician in a doctor’s practice or in a health care center. Based on the resistance pattern of individual H pylori, the physician is able to apply a resistance-adapted therapeutic regimen for eradication of the H pylori infection, if necessary after consulting a therapeutic specialist like a gastroenterologist.
The gastric string test for primary H pylori detection and isolation was first described in 1995[7], and reports on further trials have recently been published, also in the post-treatment setting[8,9]. The novelty of our approach is that we have combined the UBT and the gastric string test. This minimally invasive package for post-treatment evaluation of patients has the advantage of being more comfortable for patients, and it might be more cost-effective in most countries. In a very recent publication by Leong et al[10], a very low sensitivity of the string test was described in comparison to other published literatures. A few simple modifications of the test protocol might be responsible for the much better sensitivity in our investigation. Our patients gargled with hexitidine solution before swallowing the capsule and after withdrawing the string. Furthermore we did not squeeze the string and cultivate the obtained juice, we inoculated the string directly on a commercially available selective H pylori agar plate.
In conclusion, the combination of the UBT and the gastric string test for establishing the antibiotic susceptibility of H pylori clearly represents a valid strategy, with a possible significance in the management of H pylori infection. The implementation of this diagnostic package is not limited to a local specialized laboratory near the health care center. The availability of an inexpensive and minimally invasive antibiotic susceptibility test for H pylori is of importance both for individual patients and for monitoring the development of microbiologic resistance to standard H pylori therapy. Upper GI endoscopy can be avoided in several cases by applying this diagnostic package.
Edited by Wang XL
| 1. | Peitz U, Hackelsberger A, Malfertheiner P. A practical approach to patients with refractory Helicobacter pylori infection, or who are re-infected after standard therapy. Drugs. 1999;57:905-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Gisbert JP, González L, Calvet X, Roqué M, Gabriel R, Pajares JM. Helicobacter pylori eradication: proton pump inhibitor vs. ranitidine bismuth citrate plus two antibiotics for 1 week-a meta-analysis of efficacy. Aliment Pharmacol Ther. 2000;14:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenterol. 2001;36:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 842] [Article Influence: 36.6] [Reference Citation Analysis (1)] |
| 5. | Katelaris PH, Adamthwaite D, Midolo P, Yeomans ND, Davidson G, Lambert J. Randomized trial of omeprazole and metronidazole with amoxycillin or clarithromycin for Helicobacter pylori eradication, in a region of high primary metronidazole resistance: the HERO study. Aliment Pharmacol Ther. 2000;14:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Realdi G, Dore MP, Piana A, Atzei A, Carta M, Cugia L, Manca A, Are BM, Massarelli G, Mura I. Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter. 1999;4:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Perez-Trallero E, Montes M, Alcorta M, Zubillaga P, Telleria E. Non-endoscopic method to obtain Helicobacter pylori for culture. Lancet. 1995;345:622-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Samuels AL, Windsor HM, Ho GY, Goodwin LD, Marshall BJ. Culture of Helicobacter pylori from a gastric string may be an alternative to endoscopic biopsy. J Clin Microbiol. 2000;38:2438-2439. [PubMed] |
| 9. | Torres J, Camorlinga M, Pérez-Peréz G, Gonzalez G, Muñoz O. Validation of the string test for the recovery of Helicobacter pylori from gastric secretions and correlation of its results with urea breath test results, serology, and gastric pH levels. J Clin Microbiol. 2001;39:1650-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Leong RW, Lee CC, Ling TK, Leung WK, Sung JJ. Evaluation of the string test for the detection of Helicobacter pylori. World J Gastroenterol. 2003;9:309-311. [PubMed] |
| 11. | Leodolter A, Domínguez-Muñoz JE, von Arnim U, Kahl S, Peitz U, Malfertheiner P. Validity of a modified 13C-urea breath test for pre- and posttreatment diagnosis of Helicobacter pylori infection in the routine clinical setting. Am J Gastroenterol. 1999;94:2100-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000;45:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 13. | Breuer T, Graham DY. Costs of diagnosis and treatment of Helicobacter pylori infection: when does choosing the treatment regimen based on susceptibility testing become cost effective? Am J Gastroenterol. 1999;94:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Windsor HM, Kuteyi T, Marshall BJ. Transport medium for Helicobacter pylori collected on a string test. Helicobacter. 2003;8:490. |