Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.580
Revised: April 18, 2004
Accepted: May 29, 2004
Published online: January 28, 2005
AIM: To evaluate the efficacy of amantadine plus interferon-alpha and ribavirin in non-responder patients with chronic hepatitis C.
METHODS: Twenty-six non-responder patients received the regimen of IFN-α-2a at a dose of 6 million units three times a week, 1000-1200 mg of ribavirin daily, and 200 mg of amantadine daily in divided doses over 48 wk. After the end of treatment, at the 72nd wk, a sustained viral response rate was determined.
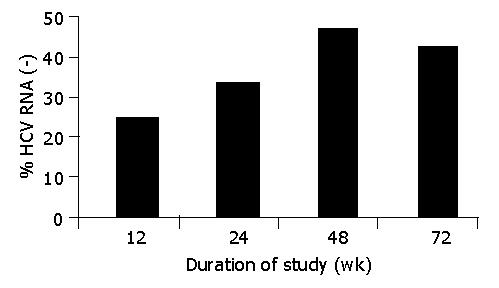
RESULTS: An early (after 12 wk of therapy) response was seen in 34.6% (9/26) of patients. Response rate at the 24th wk was 42.3% (11/26). End of treatment response (ETR) was 53.8% (14/26). Sustained viral response (SVR) was 42.3% (11/26). There was a statistically significant difference between 0 and 12 wk (P = 0.04), 0 and 24 wk (P = 0.01), 0 and 48 wk (P = 0.00), and 0 and 72 wk (P = 0.001). No patient had severe adverse effects during the treatment.
CONCLUSION: Combination regimen of interferon-α, ribavirin and amantadine can enhance sustained viral response on IFN-α and ribavirin non-responder patients with HCV. Triple therapy with amantadine should be evaluated in further studies.
- Citation: Oguz D, Cicek B, Filik L, Odemis B, Kilic M, Altintas E, Zengin N, Altiparmak E. Effect of interferon and ribavirin combined with amantadine in interferon and ribavirin non-responder patients with chronic hepatitis C (genotype 1). World J Gastroenterol 2005; 11(4): 580-583
- URL: https://www.wjgnet.com/1007-9327/full/v11/i4/580.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.580
Hepatitis C virus (HCV) affects 100-300 million people worldwide. Chronic hepatitis C is one of the leading causes of chronic liver disease and related complications such as cirrhosis and hepatocellular carcinoma[1-4]. Interferon (IFN) or IFN-containing regimens with ribavirin are still the fundamental treatment strategies for chronic hepatitis C. Ribavirin plus interferon-alpha (IFN-α) combination has led to marked advance in the treatment of IFN-α-naive or relapser patients with chronic hepatitis C, but has been shown to be only marginally effective in IFN-α-non-responders[4-8]. In these patients, combination treatment of IFN-α-2b and ribavirin reached approximately 10-25% sustained viral response (SVR)[9-11]. Amantadine is a relatively inexpensive antiviral drug with activity noted against the flaviviridae family to which the HCV belongs. Although a few early reports documented a good response to amantadine monotherapy, subsequent studies failed to confirm these results[12,13]. Pilot studies have suggested that the addition of amantadine to IFN is effective against HCV. Brillanti et al[14] reported that the combination treatment with IFN, ribavirin and amantadine did reach a relatively high sustained viral eradication rate of 48%. But, it is still debatable if amantadine alone or in combination with IFN-α and ribavirin could improve viral response in patients who failed to respond to previous combination therapy with IFN-α and ribavirin. We aimed to evaluate the safety and efficacy of IFN-α, ribavirin and amantadine in a study group of patients with chronic hepatitis C who were considered “non-responders” to previous treatment. Adverse effects developed during the therapy were also evaluated.
The Institutional Review Board of the Hospital approved this study. Adult patients with chronic hepatitis C who failed to respond to previous treatment (non-responders) were enrolled. Non-responders had not responded to induction therapy with 9 million units of IFN-α-2a daily for four weeks, followed by 3 million units three times a week for an additional 11 mo and ribavirin (1000-1200 mg in divided daily dose) (n = 27). These patients had no previous evidence of virologic response (undetectable HCV RNA level) or biochemical response (normal alanine aminotransferase level) to the combination regimen.
All patients gave informed consent, and those who agreed to participate and met the inclusion criteria were enrolled in the study. The following criteria were used to exclude patients: decompensated liver disease, immunocompromised patients or human immunodeficiency virus positivity, severe psychiatric conditions, poorly controlled diabetes mellitus, active cardiopulmonary disease, renal insufficiency, seizure disorders, autoimmune disease, uncontrolled thyroid disease, other liver diseases and pregnancy. Patients with a hemoglobin level of <130 g/L (for males), or <120 g/L (for females), platelet count <100000/mm3 and leukocyte count <3000/mm3 were also excluded. All patients were required to use an effective method of birth control during the entire study. All patients included in the study were proven to have HCV genotype 1b.
The regimen used in this study was composed of IFN-α-2a at a dose of 6 million units three times a week, 1 000-1 200 mg of ribavirin daily, and 200 mg of amantadine daily in divided doses. All potential candidates completed at least 30 d of a washout period from the last dose of their previous regimen prior to enrollment. After enrollment, patients received this regimen for 48 wk. After discontinuation of the treatment regimen, patients were subsequently followed for an additional 24 wk.
Patients were seen during treatment wk 2, 4, 8, 12, 16, and 24, and every four weeks thereafter until the end of treatment. Physical examinations were conducted during treatment wk 4, 8, and 12, and every four weeks thereafter until the end of therapy. HCV RNA levels were determined by Cobas Amplicore HCV monitor version 2.0 (threshold detection 100 copies/mL), at baseline and again at therapy wk 12, 24, 48 and 72. Viral genotype was determined at baseline. Virological assays were performed in the institution laboratory.
Liver biopsies were performed within a mo of entry into the study. Pretreatment biopsy reports were classified based on the degree of fibrosis and activity of inflammation.
Severity of adverse events, specific to interferon, ribavirin and amantadine, was recorded at each visit. Modification of IFN or ribavirin dose was not needed due to adverse events.
The primary end point of this study was a combined (biochemical and virologic) response. Patients who reached their end points (undetectable HCV RNA level and normalization of alanine aminotransferase level) were recorded as “responders”. Patients with detectable HCV RNA level during therapy or at the end of the treatment period were designated as “non-responders”. Response rates at 12th, 24th, and 48th wk (end of treatment response; ETR) and at the 72nd wk (sustained viral response) were compared. Sustained viral response (SVR) was HCV RNA clearance at 24 wk after completion of treatment.
Response rates were compared with McNemar test. A P value <0.05 was considered to be statistically significant. All analyses were performed with SPSS statistical software package.
A total of 27 patients who met the inclusion criteria were enrolled in the study. Clinicodemographic characteristics of the patients included in the study are summarized in Table 1. In this study, no patient was cirrhotic, and most had high baseline viral load (>2 000000 copies/mL) and all had genotype 1b.
| Parameters | Non-responders (n = 27) |
| Age (yr) | 45.2 |
| Gender-male (%) | 14 (50%) |
| Pretreatment HCV RNA (copies/mL) | 2195150±1575560 |
| Pretreatment ALT (IU/mL) | 142 |
| HCV genotype 1b (%) | 28 (100) |
Of the 27 patients, one decided early against treatment and dropped out, but liver biopsy result was included in the evaluation. There was no emergency condition or severe adverse effects to exclude the patients during the study. The histological activity index (HAI) and fibrosis stage of 27 patients are shown in Table 2.
| HAI Score | n (%) | Stage | n (%) |
| Minimal | 2 (7.1) | 0 | 2 (10.7) |
| Mild | 7 (25) | 1 | 7 (42.9) |
| Moderate | 11 (39.3) | 2 | 11 (10.7) |
| Severe | 7 (25) | 3 | 7 (28.6) |
An early (after 12 wk of therapy) response was seen in 34.6% (9/26) of patients. Response rate at the 24th wk was 42.3% (11/26). ETR was 53.8% (14/26). SVR was 42.3% (11/26). There was a statistically significant difference between 0 and 12 wk (P = 0.04), 0 and 24 wk (P = 0.01), 0 and 48 wk (P = 0.00), and 0 and 72 wk (P = 0.001). Figure 1 shows the ETR (48th wk) and SVR (72nd wk).
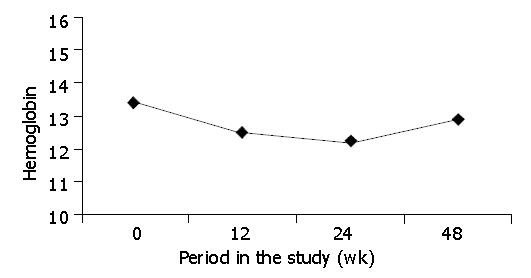
The pattern of anemia and the decline in hemoglobin levels are depicted in Figure 2. This pattern of ribavirin-induced anemia was similar to that reported in previous studies[20-23]. Overall, hemoglobin levels dropped between 10 and 15 gr/L during the first 4-8 wk after initiating treatment.
For previously untreated patients with chronic hepatitis C, treatment with interferon-alpha monotherapy resulted in a sustained viral eradication rate <20%; combining IFN-α-2b with ribavirin improved this efficacy to around 40%[9,11,15]. Despite this relatively good efficacy of IFN-α-2b and ribavirin for untreated hepatitis C patients, this regimen is not very efficacious for those who are considered “non-responders” to previous treatment. This treatment-resistant group is a major subject of concern for hepatologists because that group most likely represents the most difficult group of HCV-infected individuals to treat. Brillanti et al[14] reported that the combination treatment with IFN, ribavirin and amantadine did reach a relatively high sustained viral eradication rate of 48% in non-responders. In this study, we found that addition of amantadine to IFN-α-2a and ribavirin increased HCV RNA clearance rate in treatment of “non-responder” HCV patients.
An early virological response to IFN-α treatment is a strong predictor of SVR [2,5,6]. In our study, an early (after 12 wk of therapy) response was seen in 34.6% (9/26) of patients. All of these patients had SVR. Response rates were statistically different between the 12th, 24th, and 48th wk, suggesting that the benefit of triple therapy gradually increased with elongation of treatment, especially in the patients who do not respond early during the treatment.
Recent studies have shown nonfavorable results of triple therapy in naive patients[16-19]. Berg et al[20] claimed that amantadine should be considered a potential anti-HCV drug in future studies. But, in non-responders, it was reported that the addition of amantadine was well tolerated, and led to improvement of SVR compared with retreatment with IFN-α and ribavirin[21-23]. In contrast, our results showed hopeful response rates with an ETR of 53.8% (14/26), and SVR of 42.3% (11/26) in genotype 1.
Amantadine is an inexpensive and well tolerated drug, and our study showed that it was effective when used in combination with standard IFN-α and ribavirin[25]. Amantadine was very well tolerated by these patients. We also noted relatively low withdrawal rates compared with most of the previous studies. One patient dropped out early at the start of the study, and another refused re-treatment after biopsy. We believe amantadine does not produce any significant additional side effects.
In patients with chronic hepatitis C, one of the most effective therapies is the combination of peginterferon-alpha-2b (1.5 μg/kg per wk) plus ribavirin[17-19]. The benefit is mostly achieved in patients with HCV genotype 1 infections. Manns et al[26] showed that the SVR rate was significantly higher (42%) among patients with HCV genotype 1. The rate for patients with genotype 2 and 3 infections was about 80% in all treatment groups.
In summary, re-treatment of a strictly defined non-responder group with a 48-wk course of a triple combination regimen of IFN-α-2b, ribavirin and amantadine is associated with a sustained viral response that can not be ignored. Although our results were encouraging, further studies are needed. It is possible that alternative regimens using pegylated interferon-alpha in combination with ribavirin or a triple combination regimen (pegylated interferon, ribavirin and amantadine) may be associated with much higher rates of sustained viral eradication and lower response rates.
Assistant Editor Guo SY Edited by Kumar M and Ma JY
| 1. | Wietzkebetaraun P, Meier V, Braun F, Ramadori G. Combination of "low-dose" ribavirin and interferon alfa-2a therapy followed by interferon alfa-2a monotherapy in chronic HCV-infected non-responders and relapsers after interferon alfa-2a monotherapy. World J Gastroenterol. 2001;7:222-227. [PubMed] |
| 2. | Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 3. | Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1934] [Cited by in RCA: 1900] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 4. | Sarbah SA, Younossi ZM. Hepatitis C: an update on the silent epidemic. J Clin Gastroenterol. 2000;30:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Perrillo RP, Carey W, Jacobson IM, Payne J, Dienstag JL. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1213] [Cited by in RCA: 1130] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 6. | Gish RG. Standards of treatment in chronic hepatitis C. Semin Liver Dis. 1999;19 Suppl 1:35-47. [PubMed] |
| 7. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2434] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 8. | Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 895] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 9. | Di Bisceglie AM, Thompson J, Smith-Wilkaitis N, Brunt EM, Bacon BR. Combination of interferon and ribavirin in chronic hepatitis C: re-treatment of nonresponders to interferon. Hepatology. 2001;33:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Brillanti S, Garson J, Foli M, Whitby K, Deaville R, Masci C, Miglioli M, Barbara L. A pilot study of combination therapy with ribavirin plus interferon alfa for interferon alfa-resistant chronic hepatitis C. Gastroenterology. 1994;107:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 195] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Younossi ZM, Mullen KD, Zakko W, Hodnick S, Brand E, Barnes DS, Carey WD, McCullough AC, Easley K, Boparai N. A randomized, double-blind controlled trial of interferon alpha-2b and ribavirin vs. interferon alpha-2b and amantadine for treatment of chronic hepatitis C non-responder to interferon monotherapy. J Hepatol. 2001;34:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Smith JP. Treatment of chronic hepatitis C with amantadine. Dig Dis Sci. 1997;42:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Goff JS, Reveille RM, Johnson J. Treatment of chronic hepatitis C with amantadine. Dig Dis Sci. 2000;45:1389-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Brillanti S, Levantesi F, Masi L, Foli M, Bolondi L. Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C. Hepatology. 2000;32:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Khalili M, Denham C, Perrillo R. Interferon and ribavirin versus interferon and amantadine in interferon nonresponders with chronic hepatitis C. Am J Gastroenterol. 2000;95:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Engler S, Flechtenmacher C, Wiedemann KH, Gugler R, Stremmel W, Kallinowski B. Interferon alfa2a induction therapy in combination with ribavirin and amantadine for the treatment of naive patients with chronic HCV infection. J Viral Hepat. 2004;11:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Uyama H, Enomoto H, Kishima Y, Yamamoto M, Yoshida K, Okuda Y, Hirotani T, Kuroda T, Ito H, Matsuda M. A pilot study of combination therapy with initial high-dose interferon and amantadine hydrochloride for patients with chronic hepatitis C with the genotype 1b virus. Hepatogastroenterology. 2003;50:2112-2116. [PubMed] |
| 18. | Thuluvath PJ, Maheshwari A, Mehdi J, Fairbanks KD, Wu LL, Gelrud LG, Ryan MJ, Anania FA, Lobis IF, Black M. Randomised, double blind, placebo controlled trial of interferon, ribavirin, and amantadine versus interferon, ribavirin, and placebo in treatment naïve patients with chronic hepatitis C. Gut. 2004;53:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Angelico M, Cepparulo M, Angelico F, Francioso S, Barlattani A, Di Candilo F, Della Vecchia R, Demelia L, De Sanctis G, Gentile S. A randomized controlled trial of amantadine plus interferon-alpha2a vs. interferon-alpha2a alone in naive patients with chronic hepatitis C randomized according to the early virological response to interferon-alpha2a monotherapy. Aliment Pharmacol Ther. 2004;19:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Berg T, Kronenberger B, Hinrichsen H, Gerlach T, Buggisch P, Herrmann E, Spengler U, Goeser T, Nasser S, Wursthorn K. Triple therapy with amantadine in treatment-naive patients with chronic hepatitis C: a placebo-controlled trial. Hepatology. 2003;37:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Zilly M, Lingenauber C, Desch S, Väth T, Klinker H, Langmann P. Triple antiviral re-therapy for chronic hepatitis C with interferon-alpha, ribavirin and amantadine in nonresponders to interferon-alpha and ribavirin. Eur J Med Res. 2002;7:149-154. [PubMed] |
| 22. | Olveira A, Serrano C, Erdozain JC, Calleja JL, Castillo P, Segura JM, Escartín P. Interferon, ribavirin and amantadine in prior nonresponders to interferon and ribavirin therapy with chronic hepatitis C (genotype 1). Gastroenterol Hepatol. 2003;26:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 23. | Younossi ZM, Mullen KD, Hodnick S, Barnes DS, Carey WD, McCullough AC, Easley K, Gramlich T, Liebermann BY. Triple combination of interferon alpha-2b, ribavirin, and amantadine for treatment of chronic hepatitis C. J Clin Gastroenterol. 2003;36:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Thuluvath PJ, Pande H, Maygers J. Combination therapy with interferon-alpha(2b), ribavirin, and amantadine in chronic hepatitis C nonresponders to interferon and ribavirin. Dig Dis Sci. 2003;48:594-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Berg T, Naumann U, Wiedenmann B, Hopf U. Pilot study of interferon-alpha high-dose induction therapy in combination with ribavirin plus amantadine for nonresponder patients with chronic hepatitis C. Z Gastroenterol. 2001;39:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |