Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.561
Revised: March 17, 2004
Accepted: April 16, 2004
Published online: January 28, 2005
AIM: To investigate the effects of Danshaohuaxian (DSHX), a Chinese herbal recipe, on the apoptosis and cell cycles of hepatic stellate cells (HSCs) in rat hepatic fibrosis and its possible mechanisms.
METHODS: Seventy-six male Wistar rats were randomly divided into normal control group, hepatic fibrosis group, non-DSHX-treated group and DSHX-treated group. Except for the normal control group, rat hepatic fibrotic models were induced by subcutaneous injection of carbon tetrachloride (CCl4), drinking alcohol, giving diet of hyperlipid and hypoprotein for 8 wk. When the hepatic fibrotic models were produced, 12 rats of hepatic fibrosis group (15 rats survived, others died during the 8 wk) were sacrificed to collect blood and livers. HSCs were isolated from the other 3 rats to detect the apoptotic index (AI) and cell cycles by flow cytometry. DSHX was then given to the DSHX-treated group (1.0 g/kg, PO, daily) for 8 wk. At the same time, normal control group and non-DSHX-treated group were given normal saline for 8 wk. At end of the experiment, some rats in these three groups were sacrificed to collect blood and livers, the other rats were used for HSC isolation to detect the apoptotic index (AI) and cell cycles. Then the liver index, serum hyaluronic acid (HA) and alanine aminotransferase (ALT), degree of hepatic fibrosis, urinary excretion of hydroxyproline (Hyp) and expression of collagen types I and III (COL I and III) in these four groups were detected respectively.
RESULTS: Compared with the indexes of the hepatic fibrosis group and non-DSHX-treated group, the DSHX-treated group revealed a liver index of (0.0267±0.0017 vs 0.0423±0.0044, 0.0295±0.0019, P<0.05), levels of serum HA (200.78±31.71 vs 316.17±78.48, 300.86±72.73, P<0.05) and ALT(93.13±5.79 vs 174.5±6.02, 104.75±6.54, P<0.01), and stage of hepatic fibrosis (1.30 vs 4.25, 2.60, P<0.01) all reduced. The urinary excretion of Hyp increased (541.09±73.39 vs 62.00±6.40, 182.44±30.83, P<0.01), the COL I and III expression decreased (COL I: 1.07±0.96 vs 4.18±2.26, 3.22±1.44, P<0.01; COL III: 1.09±0.58 vs 3.04±0.62, 2.23±0.58, P<0.01), the HSCs apoptotic index of HSCs (7.81±0.47 vs 1.63±0.25, 1.78±0.4, P<0.05) and the ratio of G0-G1 phase cells increased (94.30±1.33 vs 62.27±17.96, 50.53±2.25, P<0.05). The ratios of S-phase cells (3.11±1.27 vs 9.83±1.81, 11.87±1.9, P<0.05) and G2-M phase cells (2.58±0.73 vs 23.26±10.95, 13.60±1.15, P<0.01) declined.
CONCLUSION: DSHX capsule shows certain therapeutic effects on hepatic fibrosis in rats and inhibits abnormal deposition of COL I and III in rat livers by promoting the apoptosis of HSCs and preventing their proliferation.
-
Citation: Geng XX, Yang Q, Xie RJ, Luo XH, Han B, Ma L, Li CX, Cheng ML.
In vivo effects of Chinese herbal recipe, Danshaohuaxian, on apoptosis and proliferation of hepatic stellate cells in hepatic fibrotic rats. World J Gastroenterol 2005; 11(4): 561-566 - URL: https://www.wjgnet.com/1007-9327/full/v11/i4/561.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.561
Hepatic fibrosis is an important pathological alteration occurring in various chronic liver diseases. The crucial factor of its, generation and development is the imbalance between the production and degradation of extracellular matrix (ECM) which mainly includes collagens[1]. It has been demonstrated that activated hepatic stellate cells (HSCs) are the main source of ECM[2-4], they display enhanced proliferation and synthesize unbalanced amounts of extracellular matrix proteins, matrix-degrading enzymes and their inhibitors, resulting in matrix accumulation[5]. Thus, activation and proliferation of HSCs represent the pivot of the fibrotic process[2,6]. At the same time, during the recovery of hepatic fibrosis, diminution of activated HSCs is mainly associated with apoptosis but not its phenotype transforming from activated state to quiescent state[7-9]. Consequently, inhibiting the activation and proliferation of HSCs and inducing apoptosis of activated HSCs may provide a novel therapeutic approach to the treatment of advanced hepatic fibrosis[4,7,9,10]
Danshaohuaxian (DSHX), a Chinese herbal recipe, is based on the traditional Chinese theory of removing blood stasis to promote blood circulation and nourishing the liver by removing, the obstrustion of channels, and made by several years’ clinical practice[11]. Our previous studies indicate DSHX capsule has obviously, preventive actions on hepatic fibrosis in rats[12,13] and good therapeutic effects on viral hepatitis-related hepatic fibrosis in clinical trial[11]. In the present study, we observed the effects of DSHX on the expression of collagens I and III (COL I, III) in fibrotic livers, meanwhile, the apoptotic index (AI) and cell cycles of HSCs were detected by flow cytometry to investigate its possible anti-fibrosis mechanisms in livers.
DSHX capsules, composed of five Chinese herbal medicines (tetrandrine, radix salviae miltiorrhizae, radix paeoniae rubra, astragalus membranaceus and ginkgo leaf), were produced by Guiyang Pharmaceutical Co., Guizhou, China. The procedure of production was as follows: radix salviae miltiorrhizae and ginkgo leaf were extracted with ethanol at 60-65 °C twice, then the extraction was filtered and concentrated. Afterwards, tetrandrine, radix paeoniae rubra, astragalus membranaceus and the extraction were mixed and boiled twice. Then the total extraction was filtrated and concentrated to be the unguent with a density of 1.08. Subsequently, the unguent was dealt with 950 mL/L ethanol for 24 h, then concentrated and added with farina and dried. Finally, the extraction was crumbled and granulated by 850 mL/L ethanol. The quantity standard of tetrandrine, radix paeoniae rubra, astragalus membranaceus and ginkgo leaf was controlled by thin layer chromatography (TLC). High performance liquid chromatography (HPLC) was used to detect the content of tanshinone IIA (C19H18O3) in this recipe. The content of tanshinone IIA was not less than 0.2 mg per capsule. The chemicals inside the capsules were ground before use and dissolved in distilled water to the required concentration for gavage. The hyaluronic acid (HA) kit was obtained from Beijing Northern Biological Technical Research Institute, Beijing, China. The hydroxyproline (Hyp) kit was obtained from Nanjing Jiancheng Biological Engineering Research Institute, Nanjing, China. Anti-COL I and III monoclonal antibodies were supplied by Dr. Jie Liu (Ph.D, American National Cancer Institute at NIEHS), the biotinylated goat anti-mouse IgG and Strept-avidin-biotin complex (SABC) immunohistochemical kit were supplied by Boster Biological Technology Ltd., Wuhan, China. Collogenase IV, proteinase E and Nycodenz were purchased from Sigma Chemical Co., USA. DNase I and DMEM were from Gibco (Grand Island, NY, USA). The flow cytometry (FCM) kit (DNA kit) was from BD Co., USA. All the chemicals were of the highest purity.
HITACHI 7170A automatic biochemical analytic instruments (Japan) were used. Biomias image analytic instruments were from the Image and Figure Research Institute, Sichuan University (China). Olympus BX41 microimage collecting systems (Japan) and FASC calibur clinical FCM instruments (BD Co., USA) were used.
Seventy-six male Wistar rats (200±20 g) were provided by the Experimental Animal Center of Guiyang Medical College, Guizhou, China. The rats were randomly divided into normal control group, hepatic fibrosis group, non-DSHX-treated group, DSHX-treated group, each group consisted of 19 rats.
Except for the normal control group, experimental liver fibrosis in rats was produced in every group by compound methods. The rats were subcutaneously injected with 400 g/L carbon tetrachloride (CCl4) solution (mixture of pure CCl4 and peanut oil), 0.3 mL/100 g twice a week for 8 wk (pure CCl4 was given at the first time, 0.5 mL/100 g). At the same time, the rats were fed with a hypercholesterol and hypoprotein diet (795 g/L corn farina, 200 g/L fat and 5 g/L cholesterol) every day, and 300 mL/L alcohol in drinking water every other day. The normal control group was fed with normal diet. When the liver fibrotic models were produced, 12 rats of hepatic fibrosis group (15 rats survived, others died during the 8 wk) were sacrificed to collect blood and livers. The body weights of rats were taken before sacrifice. After sacrifice, the wet livers were weighed and the same part of each rat’s liver was removed and fixed in 100 mL/L neutral formalin. The serum was centrifuged and stored at -80°C. HSCs were isolated from the other 3 rats as previously described[14-16] to detect the apoptotic index (AI) and cell cycles. DSHX was then given to the DSHX-treated group (1.0 g/kg, po, daily) for 8 wk .The doses were 16-fold of the clinical therapeutic dose. The normal control group and non-DSHX-treated group were given normal saline. At end of the experiment, some rats were sacrificed to collect livers and serum, and the others were used for HSC isolation (19 rats in normal control group, and 13 rats in non-DSHX-treated group and DSHX-treated group survived, respectively).
The index was calculated according to the formula: (rat liver weight /rat weight) ×100%.
At the eighth week of treatment, rats of hepatic fibrosis group were placed into metabolic cages to collect a 24-h urine for urinary Hyp determination. All the other groups were dealt with the same way to detect Hyp at end of the experiment.
The concentrations of serum HA and alanine aminotransferase (ALT) were respectively determined with radioimmunological (RIA) kit and the HITACHI 7170A automatic biochemical analytic instrument.
The same part (about 1 cm×1 cm×0.5 cm) of each rat’s liver was separately fixed in 100 mL/L formalin for 24 h, then processed by standard histology procedures, embedded in paraffin, cut into 5 μm thick pieces and mounted on the slide. The samples were stained with hematoxylin-eosin (HE) for general histopathology examination and Van-Gieson for evaluating the degree of liver fibrosis as described by Cheng et al[1].
The expression of collagen types I and III was observed by the SABC immunohistochemical technique. Briefly, after deparaffinization and rehydration, the histological sections were treated with 30 mL/L H2O2 for 10 min, subjected to compound proteinases for 10 min, treated with normal goat serum for 20 min, then incubated with anti-collagen type I or III monoclonal antibody for 20 h at 4 °C. Afterwards, sections were washed with PBS and incubated with the biotinylated goat anti-mouse IgG for 20 min at 37 °C. Then sections were washed with PBS and treated with SABC. Subsequently, the sections were stained with 3’-3’-diaminobenzidine (DAB). The positive areas were tan. For each liver sample, negative controls were performed on the adjacent sections, replacing the primary antibodies with PBS. The sections were counterstained with hematoxylin, dehydrated in graded alcohols, and mounted. Finally, 5 low power fields were chosen randomly in each section and the images were analyzed by the Biomias image analytic instrument to examine the percentage of the positive staining area in each section.
The indexes were detected according to the FCM kit manufacturer’s instructions. Briefly, HSCs were treated with Triton X-100 and RNase respectively, and then stained with 65 g/L propidium iodide (PI) at 4 °C for 1 h. After being put in darkness for 30 min, apoptotic index and cell cycles were observed. At the end, the data were put into HP-300 consort 30 computer and dealt with single histogram statistics software.
Quantitative data were expressed as mean±SD, and subjected to one-way analysis of variance (ANOVA) followed by t test for multiple comparisons. Ordinal data were analyzed by Ridit analysis. P<0.05 was considered statistically significant.
As shown in Table 1, compared with the normal control group, the liver index (relative liver weight) of hepatic fibrosis group was significantly increased (P<0.01), while those of DSHX-treated group and non-DSHX-treated group were obviously decreased as compared with hepatic fibrosis group (P<0.01), especially the DSHX-treated group.
As shown in Table 1, a significant increase in urinary Hyp excretion was observed in the normal control group compared with the DSHX-treated group. The amount of urinary Hyp excretion in the hepatic fibrosis group and non-DSHX-treated group was obviously higher compared to the normal control group (P<0.01), while that in DSHX-treated group was respectively 9-fold and 3-fold of that in the hepatic fibrosis group and non-DSHX-treated group.
As shown in Table 2, the concentrations of serum HA and ALT in hepatic fibrosis group and non-DSHX-treated group markedly increased compared with those in the normal control group, while those were evidently lower in DSHX-treated group than in the hepatic fibrosis group and non-DSHX-treated group.
HE and V-G staining showed that the hepatocytes of normal control group arrayed radially with the central vein and there was no regenerating collagen fiber. After treated with CCl4 for 8 wk, the lobular structure of hepatic fibrosis group was destroyed and the hepatic plates were in disorder. Furthermore, the fibrous connective tissues with a lot of inflammatory cells regenerated in portals. Meanwhile, the collagen fibers expanded into the hepatic parenchyma, and fibrous septa surrounding and separating the normal lobules formed. The degree of hepatic fibrosis in this group significantly increased compared with that in the normal control group (P<0.01). After CCl4 treatment for 8 wk and normal saline treatment for another 8 wk, though hepatic fibrosis in non-DSHX-treated group was alleviated compared with that in the CCl4-induced hepatic fibrosis group, the fibrous septa expanding in the former group was still obvious, the pseudo lobules even existed in severe samples and the average degree of fibrosis was still obviously higher compared to normal group (P<0.01). The lobular structure of the DSHX-treated group significantly improved, the fibrous connective tissue regeneration decreased and the fibrous septa were thinner and less obviously compared with CCl4-induced hepatic fibrosis group and non-DSHX-treated group. The details about the degree of hepatic fibrosis in each group are shown in Table 3.
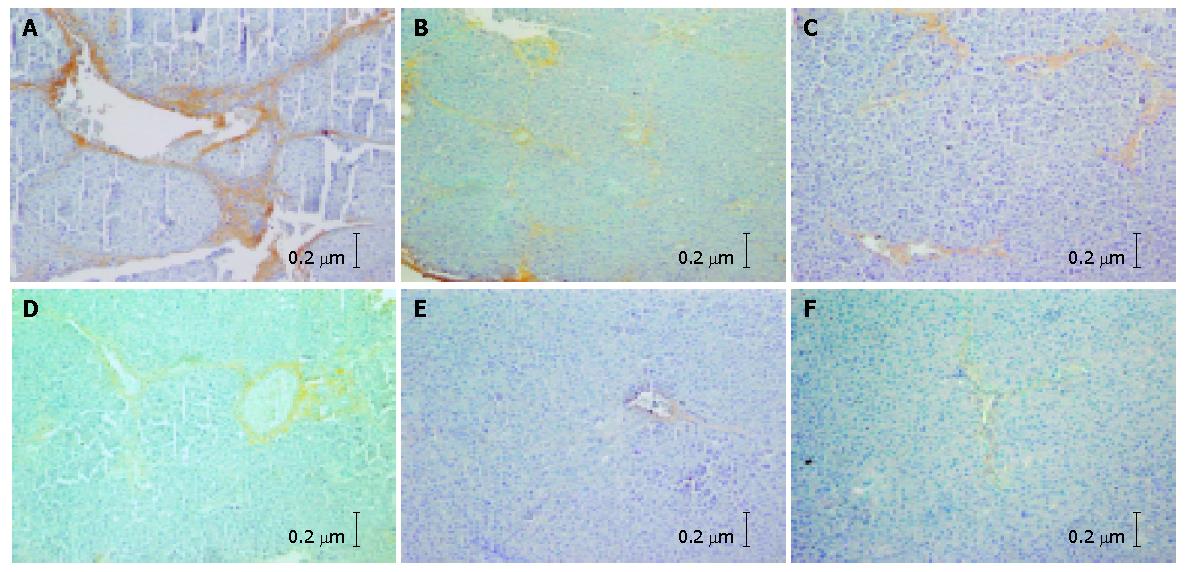
With the SABC immunohistochemical technique, only sliqht yellow COL I and III fibers could be observed under microscopy in the normal control group. The COL I and COL III fibers were mainly distributed in portals and around the central veins. In the hepatic fibrosis group, a great deal of tan COL I and COL III fibers were detected in portals and hepatic sinusoids. The collagen fibers expanded into the liver parenchyma and the fibrous septa formed. Hepatocytes were surrounded by fibrous septa and the pseudo lobules formed (Figures 1A and B). The percentages of COL I and COL III in this group were significantly higher than those in the normal control group (P<0.01). In the non-DSHX-treated group, the expression of COL I and III was ameliorated compared with the hepatic fibrosis group, while the tan collagen was still distributed extensively and the pseudo lobules also could been seen (Figures 1C and D). The collagen percentage was also markedly higher in this group than in the normal control group (P<0.01). The percentages of COL I and COL III in the DSHX-treated group were obviously lower than those in the hepatic fibrosis group and non-DSHX-treated group. There was no pseudo lobule formed in the DSHX-treated group (Figures 1E and F, Table 4).
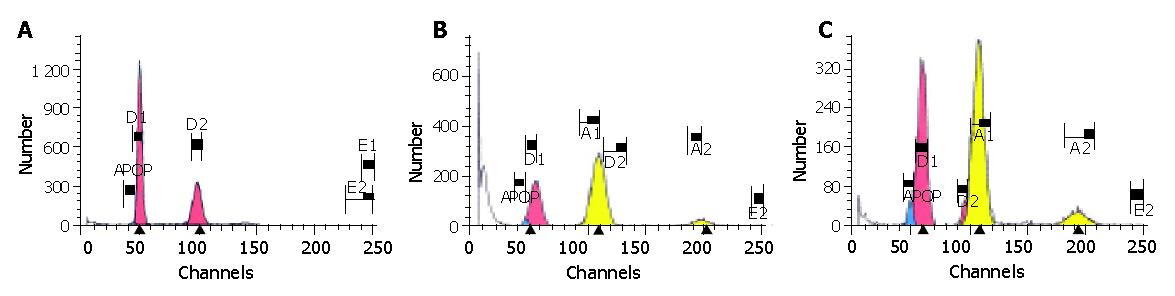
The results are shown in Table 5 and Figure 2.
As shown in Figure 2, there were only a few apoptotic cells (0.28%) before the phase of diploid G0-G1 in the normal control group and the cell cycle was complete; while the evident apoptosis peak and aneuploid peak with different degrees could be seen in the hepatic fibrosis group, non-DSHX-treated group and DSHX-treated group. The AI in hepatic fibrosis group was increased to 1.63%, and after treated with DSHX for 8 wk, the AI in DSHX-treated group was increased to 7.81%, which was markedly higher than that in the hepatic fibrosis group and non-DSHX-treated group (Table 5). Meanwhile, the percentages of S-phase cells in hepatic fibrosis group and non-DSHX-treated group obviously increased as compared with the normal control group, while decreased obviously after treated with DSHX for 8 wk. Furthermore, compared with the hepatic fibrosis group, non-DSHX-treated group and normal control group, the cells in G0-G1 phase in DSHX-treated group markedly increased, while the cells in G2-M phase decreased.
Liver fibrosis is characterized by an increase in the synthesis and deposition of ECM, especially the proliferation and deposition of collagens[17]. When hepatic fibrosis occurs, proliferated collagens mainly including COL I and III account for 50% of the total protein in fibrotic liver[10,18,19], and collagens are the main components of ECM. Therefore, COL I and III are the important parameters reflecting the metabolism of collagens in liver. In the present study, we observed alterations of the expression of COL I and III in hepatic fibrotic rats after they were treated with DSHX for 8 wk. The results showed that after treated with DSHX, the expression of COL I and III in fibrotic livers of rats was significantly lower, the fibrous septa became thinner and were distributed lightly. At the same time, the urinary excretion of Hyp, a unique component of collagenous amino acids, increased obviously. Meanwhile, we found in the DSHX-treated group that the liver index, and serum HA and ALT levels were declined, thereby decreasing the degree of hepatic fibrosis. These results demonstrate that DSHX capsule might have certain therapeutic effects on hepatic fibrosis of rats by improving the liver function and inhibiting the abnormal deposition of ECM in liver.
Recent researches[20-25] have demonstrated that once HSCs are activated, their functions would alter greatly, such as excretion a great deal of cytokines and tissue inhibitors of metalloproteinase (TIMPs), etc. These alterations could induce liver fibrosis directly. Moreover, activated HSCs are the main source of ECM when liver fibrosis occurs[1-3,26,27], while the diminution of HSCs is mainly associated with apoptosis occurred in activated HSCs[7-9,28,29]. Accordingly, inhibiting activation and proliferation of HSCs and inducing apoptosis of the activated HSCs have become the important methods against the liver fibrosis. In the present study, we observed that S-phase HSCs in the hepatic fibrosis group and non-DSHX-treated group evidently increased compared with those in the normal control group, while after treated with DSHX for 8 wk, S-phase HSCs in the DSHX-treated group declined obviously compared with those in the two former groups. These results imply that DSHX capsule could inhibit the proliferation of HSCs. Meanwhile, the ratio of G0-G1 phase cells in DSHX-treated group significantly increased, suggesting that through inhibiting transformation of HSCs from G0 phase to G1 phase, DSHX capsule could prevent HSC proliferating. In addition, there were only a few apoptotic HSCs in the normal control group, while the apoptotic index in hepatic fibrosis group and especially in non-DSHX-treated group was higher than that in the normal control group. These results show that there is spontaneous apoptosis in livers during the recovery from injury. Furthermore, the apoptotic index in DSHX-treated group was much higher than that in hepatic fibrosis group and non-DSHX-treated group.
These findings suggest that DSHX capsule could improve liver functions and degrade COL I and III, and these actions are related inhibiting the proliferation of HSCs and inducing the apoptosis of activated HSCs. The associated mechanisms might be the ability of DSHX to down-regulate the expression of COL I and III mRNA by preventing HSCs from proliferating and diminishing the number of activated HSCs. In addition, this capsule could indirectly decrease the abnormal deposition of ECM by declining the expression of TIMP, plasmin activator inhibitor-1 (PAI-1), etc, in liver to inhibit their actions on collagenases and promote the absorption of COL I and III. Our previous study demonstrated that[30] DSHX capsule could prevent the expression of transforming growth factor β1 (TGF-β1) in fibrotic rat livers. It has been reported that TGF-β1[6] could directly and indirectly promote the proliferation of HSCs through multiplying the expression of platelet derived growth factor receptors (PDGFRs) and the combination of platelet derived growth factors (PDGF) and PDGF-R[31]. Consequently, we may further suppose that DSHX capsule can reduce the transformation and synthesis of TGF-β1 to restrain the proliferation of HSCs, thereby controlling the abnormal deposition of ECM, which mainly includes COL I and III and inhibits hepatic fibrosis. The mechanisms of how the capsule stimulates apoptosis of HSCs need further study.
Assistant Editor Guo SY Edited by Kumar M and Wang XL
| 1. | Cheng ML, Yang CQ. Basic study and clinical research on hepatic fibrosis. 1st. Los Angeles: First Jumbo Publishing Co 2002; 16-30. |
| 2. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] |
| 3. | Williams EJ, Iredale JP. Liver cirrhosis. Postgrad Med J. 1998;74:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | Jiang CM, Liu C, Liu P. The developing researches of the HSC during liver fibrosis. Zhonghua Xiaohua Zazhi. 2000;20:258-260. |
| 7. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 828] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 8. | Rippe RA. Life or death: the fate of the hepatic stellate cell following hepatic injury. Hepatology. 1998;27:1447-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Saile B, Knittel T, Matthes N, Schott P, Ramadori G. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997;151:1265-1272. [PubMed] |
| 10. | Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Cheng ML, Ding YS, Luo YF, Tian M, Wu J, Luo TY, Wu YY, Liu Q, Yang J. The clinical research of the therapeutic effects of Han-Dan-Bi-Tuo on chronic active hepatitis. Zhongguo Zhongxiyi Jiehe Zazhi. 1996;16:431-432. |
| 12. | Li CX, Luo J, Li L, Yang WX, Lei TW, Huang NH, Cheng ML. The protective effects of Han-Dan-Gan-Ge on CCl4-induced liver fibrosis in rats. Zhongguo Gonggong Weisheng. 1998;14:527-529. |
| 13. | Zhang HN, Li CX, Huang NH, Luo J, Cheng ML. The preventive actions of traditional Chinese medicine Han-Dan-Gan-Le on porcine serum-induced liver fibrosis in rats. Guizhou Yiyao. 2001;25:415-417. |
| 14. | Luo Y, Dai LL, Shen DM, Yao YQ, Zhang DZ, Wang B. The isolation and culture of rat hepatic stellate cells with collagenase in situ liver recirculating perfusion. Chongqing Yike Daxue Xuebao. 2002;27:48-52. |
| 15. | Zhang YJ, Yang XS, Wu PS, Liu SR. A simple and economic method for isolating hepatic lipocytes. Diyi Junyi Daxue Xuebao. 2001;21:304-306. |
| 16. | Zhu YH, Hu DR. The establishment and application of the HSC system. Shijie Huaren Xiaohua Zazhi. 1999;7:348-349. |
| 17. | Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 886] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 19. | Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193-203. [PubMed] |
| 20. | Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315-334, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 22. | Eng FJ, Friedman SL. Transcriptional regulation in hepatic stellate cells. Semin Liver Dis. 2001;21:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Gaça MD, Zhou X, Benyon RC. Regulation of hepatic stellate cell proliferation and collagen synthesis by proteinase-activated receptors. J Hepatol. 2002;36:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Caligiuri A, De Franco RM, Romanelli RG, Gentilini A, Meucci M, Failli P, Mazzetti L, Rombouts K, Geerts A, Vanasia M. Antifibrogenic effects of canrenone, an antialdosteronic drug, on human hepatic stellate cells. Gastroenterology. 2003;124:504-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Gong W, Pecci A, Roth S, Lahme B, Beato M, Gressner AM. Transformation-dependent susceptibility of rat hepatic stellate cells to apoptosis induced by soluble Fas ligand. Hepatology. 1998;28:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Zhang WS, Cheng ML, Lu YY. Effect of HanDanGanLe on the cytokines in fibrotic rats. Zhonghua GanZangBing ZaZhi. 2003;11:285-287. [PubMed] |
| 31. | Liu F, Liu JX. The actions of TGF-β1 in the hepatic fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:86-88. |