Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.529
Revised: April 18, 2004
Accepted: May 9, 2004
Published online: January 28, 2005
AIM: To determine the expression of c-fos in gastric myenteric plexus and spinal cord of rats with cervical spondylosis and its clinical significance.
METHODS: A cervical spondylosis model was established in rats by destroying the stability of cervical posterior column, and the cord segments C4-6 and gastric antrum were collected 3, 4 and 5 mo after the operation. Rats with sham operation were used as controls. c-fos neuronal counter-staining was performed with an immunohistochemistry method. Every third sections from C4-6 segments were drawn. The 10 most labeled c-fos-immunoreactive (Fos-IR) neurons were counted, and the average number was used for statistical analysis. The mean of Fos-IR neurons in myenteric plexus was calculated after counting Fos-IR neurons in 25 ganglia from each antral preparation, and expressed as a mean count per myenteric ganglion.
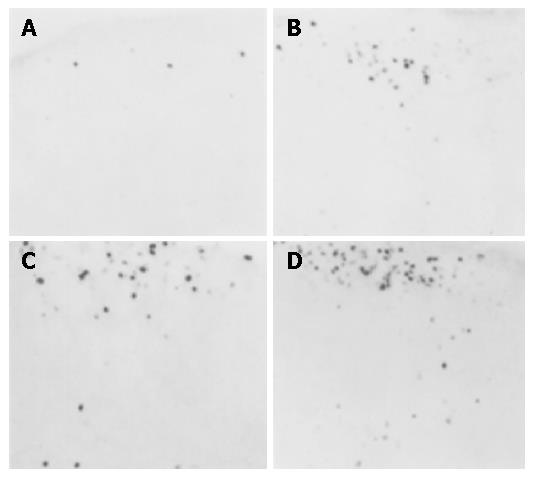
RESULTS: There were a few c-fos-positive neurons in the cervical cord and antrum in the control group. There was an increased c-fos expression in model group 3, 4 and 5 mo after operation, whereas there was no significant increase in c-fos expression in the control group at 3, 4 and 5 mo. More importantly, there was a significant difference in c-fos expression between rats followed up for 3 mo and those for 5 mo in the model group (11.20±2.26 vs 27.68±4.36, P<0.05, for the cervical cord; and 11.3±2.3 vs 29.3±4.6, P<0.05, for the gastric antrum). There was no significant difference between rats followed up for 3 mo and those for 4 mo and between rats followed up for 4 mo and those for 5 mo in the model group.
CONCLUSION: c-fos expression in gastric myenteric plexus was dramatically associated with that in the spinal cord in rats with cervical spondylosis, suggesting that the gastrointestinal function may be affected by cervical spondylosis. If this hypothesis is confirmed by further studies, functional gastrointestinal diseases such as functional dyspepsia and irritable bowel syndrome could be explained by neurogastroenterology.
- Citation: Song PS, Kong KM, Niu CY, Qi WL, Wu LF, Wang XJ, Han W, Huang K, Chen ZF. Expression of c-fos in gastric myenteric plexus and spinal cord of rats with cervical spondylosis. World J Gastroenterol 2005; 11(4): 529-533
- URL: https://www.wjgnet.com/1007-9327/full/v11/i4/529.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.529
c-fos is a proto-oncogene or a cellular oncogene, which exists extensively in genomes of eucaryotes. Most of them turn into active oncogenes, which are called viral oncogenes by reverse transcription of virus. c-fos plays a key role in life, since it participates in normal cell growth, proliferation and regulates message transfer in cells. During the last ten years, most studies have focused on c-fos expression that is induced by the controlled and natural irritations[1-3], most of which are acute, and only a few are chronic. At present, physiological and pathophysiological mechanism are deduced from these studies on c-fos[4-10]. For example, cervical spondylosis is caused by cervical disc degeneration, and subsequent facet joint degeneration. Symptoms occur when the spinal cord, nerves and blood vessels are injured or irritated. Chronic epidural scar adhesion and substance P are related with c-fos expression[11]. Expression of c-fos protein has been found in the herniated discs[12], which participates in regulating AP-1 in extracellular matrix. Some studies have proven that c-fos is associated with gastrointestinal diseases[5,6,9,13]. However, there has been no report on c-fos expression in gastric myenteric plexus and the spinal cord in cervical spondylosis. In the present study, we determined the simultaneous changes of c-fos expression in gastric and spinal cord induced by cervical spondylosis and its clinical significance.
Ninety-six 4-mo-old Sprague Dawley rats, weighing 350±40 g, purchased from the Animal Laboratory of the First Military Medical University, Guangzhou, China, with the approval of the Institutional Animal Care and Use Committee of the Shantou University Medical College, were randomly divided into model and control groups. The rats were fed a normal diet, with 8 in one big cage, and kept for 3, 4 and 5 mo, respectively, after the experimental or sham operations as described below. Each group consisted of 8 male and 8 female rats at each time point.
The rats in model group were anesthetized by injecting intraperitoneally 40 mg/kg of pentobarbital sodium. Dorsal neck was shaved and a longitudinal incision about 2.5 cm was followed. The dorsal muscles were separated and reserved. Spinal processes, inter spinal ligaments, part of superior and inferior articular processes between C3-7 levels were cut off. When the movement degree between the neighboring superior and inferior laminae was increased obviously after operation, the incision was then closed. The successful models were confirmed by evaluating X-ray films and the motion function with oblique board test 3, 4 and 5 mo, respectively, after the operation[14]. For rats in the control group (sham operation group), the incision was closed after opening without further operation.
The rats were euthanized with a lethal dose of pentobarbital sodium (100 mg/kg) followed immediately by intracardiac perfusion with 100 mL of 0.1 mol/L PBS (pH 7.4), and 400-500 mL of fixative (4% paraformaldehyde, 0.05% picric acid in PBS, 0.15 mol/L, pH 7.4). Cervical cords were removed 1 h later and post-fixed in the same fixative for an additional 3 h. After cryoprotection overnight in 30% sucrose in 0.1 mol/L PBS, 50 µm-frozen-sections were cut in the transverse plane and collected in 0.05 mol/L PBS for immunocytochemical analysis.
All immunohistochemical reactions were performed at room temperature on floating sections agitated on a shaker. Sections were incubated for 2 h in 3% normal goat serum and 0.3% Triton X-100 in 0.15 mol/L PBS (NGS-T-PBS) and incubated overnight in a rabbit polyclonal anti-c-fos antibody (Sigma, Genetimes Technology, Inc.) diluted in NGS-T-PBS (1:100000). After being washed in 0.15 mol/L PBS and incubated for 1 h in a biotinylated goat anti-rabbit antibody (1:500 in NGS-T-PBS Sigma), sections were washed again in 0.15 mol/L PBS and incubated for 2 h in avidin-biotin peroxidase complex (ABC Elite; Sigma, Genetimes Technology, Inc.) and stained for 2 min in a solution containing 0.05% of 3,3’ -diaminobenzidine (DAB) and 0.2% ammonium nickel sulfate in 0.15 mol/L Tris buffer, adding doses of H2O2 for 5 min to obtain the following dilutions: 0.001, 0.005, 0.015, 0.025, and 0.075%. Finally, the reaction was stopped by washing in Tris buffer, the sections were mounted on gelatin-coated slides under microscope.
c-fos-immunoreactive (Fos-IR) neurons were analyzed throughout the C4-6 spinal segments. Sections were first drawn with a camera lucida, noting the boundaries between the white and gray matter and the reticular part of the lamina. Under bright-field microscope (objective 20×), each Fos-IR neuron was drawn on the general boundary drawings obtained at the former step. Every third section from the C6-4 segments was drawn, and Fos-IR neurons were counted. The average from the 10 most labeled sections of the C4-6 segments was used for further statistical analysis.
Rats were euthanized with a lethal dose of pentobarbital sodium, and when fully unresponsive, they were transcardially perfused with 100 mL of 0.1 MPBS. (pH 7.4), and followed by 500 mL of fixative (4% paraformaldehyde, 0.05% picric acid in PBS). The stomach was then extracted and opened at the greater curvature, thoroughly rinsed, and post-fixed for 24-48 h with the same fixative. Before cutting, tissue samples were blocked and immersed for 24 h in 25% sucrose in PBS for cryoprotection. The tissue blocks were then mounted on a chuck using an embedding gel and quickly frozen. Sections (20-25 µm thick) were cut in a cryostat. The method for neuronal counter-staining was adapted[15,16]. Briefly, the sections were first treated with hydrogen peroxide (3% H2O2 and methanol, 1:4) for 20 min at room temperature followed by three washes with PBS. After a brief rinse in distilled water the sections were stained for 2 h at 42 °C in cuprolinic blue (0.3% quinolinic phthalocyanine in 0.05 mol/L sodium acetate-1.0 mol/L magnesium chloride buffer, pH 4.9). They were then rinsed briefly in distilled water, differentiated for 2 min in the above buffer, and finally washed three times in PBS. For subsequent c-fos staining the sections were immersed in a blocking solution (5% normal goat serum with 1% BSA, 0.5% Triton X-100 in PBS) for 2 h before with rabbit c-fos polyclonal antibody diluted in NGS-T-PBS (1:50000). The sections were rinsed four times in PBS-Gel (0.1% gelatin) and then incubated for 1h in a biotinylated goat anti-rabbit antibody (SIGMA, 1:500 in NGS-T-PBS). After three rinses in PBS-gel, the sections were incubated for 1 h in an avidin-biotin complex (1:500, Vectastain ABC Elite Kit, Sigma, Genetimes Technology, Inc.). The antibody complex was then rinsed again three times in PBS and visualized using a metal-enhanced 0.05% of 3,3’-diaminobenzidine (DAB) for 3-5 min, resulting in a dark blue-black nuclear stain. Fos-IR cells were counted under microscope in 25 ganglia from each antral preparation and expressed as a mean percentage count per myenteric ganglion. Myenteric ganglia were recognized as clearly delineated groups of neurons separated by well-defined internodal fiber tracts. The mean from all animals in each group was used to calculate the group mean. Data are expressed as mean±SD of the number of cells or neurons per ganglion.
The number of Fos-labeled neurons was determined without knowledge of treatment. Ten sections from each rat cord were quantified. Data were presented as mean±SD, statistical comparisons were made by analysis of variance (ANOVA) or Student’s t test, P<0.05 was considered statistically significant. Comparisons of mean values between groups were performed with one-way ANOVA, followed by Duncan’s contrast.
There were only a few c-fos expressing neurons in the cervical cord of the control group. However, increased c-fos expression was observed in the model groups 3, 4 and 5 mo after the operation (Figure 1, Table 1). There was no significant spontaneous c-fos expression in the spinal cord in the control group 3, 4 and 5 mo after sham operation. However, there was significant increase in c-fos expression in model group. More importantly, there was significant difference in c-fos expression between rats kept for 3 mo and those for 5 mo in the model group (P<0.05) (Table 1).
| Group | Number of c-fos positive neurons at different timepoints | ||
| 3 mo | 4 mo | 5 mo | |
| Control | 1.25±0.25 | 2.03±0.50 | 1.98±0.60 |
| Model | 11.20±2.26 | 15.02±3.91 | 27.68±4.36 |
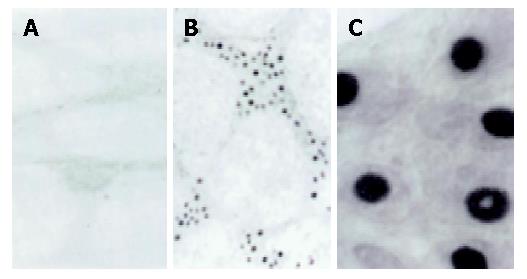
The number of fos-positive neurons in the gastric antrum was expressed as a percentage of the total number of neurons in per ganglion as assessed by cuprolinic blue counterstaining. There was no significant spontaneous c-fos expression in the antrum in the control group 3, 4 and 5 mo after sham operation. However, there was significant increase in c-fos expression in model group (Figures 2, 3, Table 2). More importantly, there was significant difference in c-fos expression between rats kept for 3 mo and those for 5 mo in the model group (P<0.05)(Table 2).
| Group | Number of c-fos positive neurons in antral ganglia at different time points | ||
| 3 mo | 4 mo | 5 mo | |
| Control | 2.4±0.6 | 2.8±0.7 | 3.2±0.8 |
| Model | 11.3±2.3 | 19.1±3.2 | 29.3±4.6 |
c-fos protein, as a product of the immediate early gene[17], has been regarded as an abnormal sign of neurons after injury or irritation[18,19]. Normally, c-fos is expressed at a lower level in most cells including neurons, and participates in growth, differentiation, signal transferring and plasticity changes of nerve cells. It also plays a role in some physiological courses of learning and memory. In pathological conditions, expression and regulation of c-fos are associated with many diseases, such as spine cord injury[20], nerve injury[8,9], burn[4], drug stimulation[2,6] and disc degeneration[12].
Previous studies on c-fos expression have concentrated on acute irritations, such as control and natural stimulation. In a study, c-fos was expressed positively in cord and hypothalamus, after rat post claws were clamped for 1-5 min each time and continued for 4 h[1]. Dense labeled Fos-like immunoreactivity in the superficial dorsal horn (laminae I and II) and in the neck of the dorsal horn was found when awakened rats were injected subcutaneously 150 µL of 5% formalin into the plantar aspect of the right hindpaw[2]. Kominato et al[3] observed c-fos expression in the spinal cord dorsal horn neurons, indicating that responses/activation by the noxious stimulation applied to the periphery are elevated in spinal cord neurons in neuritis model of the lumbar nerve root induced by disc. c-fos-neurons (neurons with c-fos protein-like immunoreactivity) following stimulation of the cutaneous receptive fields have been found in laminae I and II of the first and second cervical segments of the spinal cord ipsilateral to the stimulation, and exhibit a clear somatotopic segregation, i.e., the c-fos-neurons responding to receptive fields in the mandibular, maxillary, and ophthalmic divisions are distributed in a mediolateral sequence. On the other hand, c-fos-neurons responding to the oral mucous membrane have been found in the similar laminae of the rostral pole of subnucleus caudalis bilaterally. They are all found in most dorsal subnuclei without segregation. For example, topographic distributions of c-fos-neurons resulting from the mandibular and maxillary gingival stimulations are indistinguishable[21]. Noxious somatic stimulation induces expression of c-fos-like immunoreactivity in catecholaminergic neurons with habenular nucleus projection in the medullary visceral zone of rats[22]. The c-fos expressing level increases significantly in pathological habitus than in normal controls[4].
Recent studies have proven that c-fos expression is closely related to gastrointestinal diseases[23-29]. For example, increased neuronal expression of c-fos protein in the brain has been observed after intravenous injection of gastrin in rats, which reveals a close relationship between gastrin and c-fos protein expression[5]. Injection of cocaine and amphetamine-regulated transcript peptide (CARTp) into the 4th ventricle strongly suppresses sucrose drinking and stimulates expression of c-fos in the solitary tract (NTS)[6].
Gastric irritation induces the c-fos expression in center nerve system (CNS). Vrang et al[7] reported that gastric balloon distension (1.4 mL/min for the first 5 min, 0.4 mL/min for the next 5 min, 9 mL total, held for 60 min) in nonanesthetized, freely moving rats produced 12- and 17-fold increases in c-fos- expression in NTS neurons when distension was mainly in the fundus or corpus, respectively. Neuronal excitation in the NTS and area postrema induces expression of c-fos messenger RNA after intragastric administration of HCl (0.35-0.7 mol/L) as demonstrated by in situ hybridization autoradiography, but subdiaphragmatic vagotomy suppresses the c-fos mRNA response to intragastric acid[8]. In a previous study, noxious gastric distention to 80 mmHg induced a mean of 724 c-fos- immunoreactive nuclei per section[9]. After subdiaphragmatic vagotomy plus 80 mmHg distention, the mean of c-fos- immunoreactive nuclei reduced to 293 per section, whereas spinal transection at T2 plus 80 mmHg distention induced the mean of 581 nuclei per nucleus of the solitary tract section. From the above studies, we would deduce that noxious gastrointestinal irritation is a signal by vagus to the NTS and lumbosacrum cord, and a signal by the sympathetic nerve to thoracic cord, where c-fos expressions is induced.
Many studies have shown that c-fos expression is related to functional gastrointestinal diseases, such as c-fos abnormal expression in intestinal myenteric plexus and CNS in inflammatory bowel disease (IBD), irritable bowel syndrome (IBS) and Crohn’s disease[30,31]. Enteric nervous system (ENS) owns a strong biological compatibility, and c-fos may be a good index of ENS to short and/or chronic gastrointestinal irritation[32].
Because of the accumulated knowledge of diseases, there is a rapid transition in modern medical sciences from the simple biological pattern based on unity biology to the biology-psychology-society pattern. The traditional “functional gastrointestinal disorders” in the past, are now known as multiple physical and pathophysiological diseases involving gastrointestinal motility, gut sensitivity, brain-intestine axis, brain-intestine peptides, society-physiology factors and stress. Especially, the conception of brain-intestine axis and neurogastroenterology has been put forward, which indicates that the alimentary canal is controlled by both the sense and motion nerves. When the nerve is injured, the lesion will impact on both the sense and motion realms. Even though the lesion is limited in only one realm, the change will result in the change of other realms’ function[33,34]. Theoretically, only when gastrointestinal and CNS are integratively investigated, can the multiple physical and pathophysiological diseases be further understood.
Chronic irritation also induces c-fos expression. The chronic epidural scar adhesion has been reported to be related to the expression of substance P and c-fos gene in the spinal cord[11]. c-fos expression has been found in the herniated intervertebral disc tissue, and c-fos regulates and participates in the expression of activating protein (AP-1) in extracellular matrix[12]. Among the cervical diseases, the disorders of functional sympathetic nerve have been taken much more cares, which include about 20 kinds of diseases or symptom-groups, such as blood pressure disorder, cardiac arrhythmia, dizziness, eyesight malfunction and gastrointestinal function[35,36].
In a study on the inhibitory pathway of CNS, Blackshaw et al[37] found that gamma-aminobutyric acid (GABA) B receptor-mediated effects on vagal pathways were involved in vago-vagal reflex transmission.
The clinical symptoms of cervical spondylosis include gastrointestinal disorders resulted from sympathetic type of cervical spondylosis. One mechanism is that when the sympathetic nerve is irritated or injured by disc degeneration and facet joint disorders due to osteophytes and cervical muscle overexertion and injury, the irritation reaches the brain cortex by nerve reflex and produces a higher or lower sympathetic irritability, and then multiple dysfunction of the neck, upper limbs and gastrointestine, which is called neck-stomach syndrome. Another mechanism is that sympathetic never and spinal nerve root branches are irritated by degenerated cervical and thoracic disc, which further affects gastrointestinal reflex[36].
The mechanism of functional dyspepsia (FD) and IBS is associated with CNS[33,38]. Our study has confirmed that the longer the cervical vertebra is degenerated, the more the c-fos protein is expressed. Therefore, we suggest that there is a direct or indirect relationship between the neck and stomach, and that it is the sympathetic nerve that results in the c-fos expression both in spinal cord and in gastric myenteric plexus in cervical spondylosis. Indeed, many studies have proven that sympathetic nerve fibers distribute around the dorsal root ganglion (DRG) neurons, and the fibers interact with these neurons[39,40].
The c-fos expression in gastric myenteric plexus is dramatically associated with that of spinal cord of rats with cervical spondylosis and the gastrointestinal function may be affected by cervical spondylosis. I this hypothesis is confirmed by further studies, some functional gastrointestinal diseases such as FD and IBS could be explained by neurogastroenterology.
Edited by Xia HHX and Wang XL Proofread by Chen WW
| 1. | Bullitt E. Induction of c-fos-like protein within the lumbar spinal cord and thalamus of the rat following peripheral stimulation. Brain Res. 1989;493:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 185] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Presley RW, Menétrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323-335. [PubMed] |
| 3. | Kominato Y, Tachibana T, Dai Y, Tsujino H, Maruo S, Noguchi K. Changes in phosphorylation of ERK and Fos expression in dorsal horn neurons following noxious stimulation in a rat model of neuritis of the nerve root. Brain Res. 2003;967:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Xiong JX, Li HD, Gong FY, Lu JH. Effect of severe burn on expressions of c-fos and somatostatin in brain and their relationship with brain edema in rats. Acta Acaodemiae Medicinae Militaris Tertiae. 2002;24:760-763. |
| 5. | Yakabi K, Iwabuchi H, Nakamura T, Endo K, Fukunaga Y, Kumaki I, Takayama K. Neuronal expression of Fos protein in the brain after intravenous injection of gastrin in rats. Neurosci Lett. 2002;317:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Zheng H, Patterson LM, Berthoud HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res. 2002;957:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470-R478. [PubMed] |
| 8. | Schuligoi R, Jocic M, Heinemann A, Schöninkle E, Pabst MA, Holzer P. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996;74:873-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Ueyama T, Saika M, Senba E. Distinct gene expression in the stomach following stress and alcohol exposure. Am J Physiol Gastrointest Liver Physiol. 2000;279:G73-G81. |
| 11. | Zhang ZW, Xiao G, Zhu D, Yan JJ, Xu XX. The epidural scar adhesion and the expression of substance P and c-fos gene in the spinal cord. Chin J Mod Med. 2001;11:6-8. |
| 12. | Tolonen J, Grönblad M, Virri J, Seitsalo S, Rytömaa T, Karaharju E. Oncoprotein c-Fos and c-Jun immunopositive cells and cell clusters in herniated intervertebral disc tissue. Eur Spine J. 2002;11:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Yuan PQ, Taché Y, Miampamba M, Yang H. Acute cold exposure induces vagally mediated Fos expression in gastric myenteric neurons in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G560-G568. [PubMed] |
| 14. | Song PS, Kong KM, Qi WL, Wang XJ, Han W, Huang K, Chen ZF. Compare study on the models of cervical spondylosis of muscle imbalance and posterior column instability in rats. Shantou Daxue Yixueyuan Xuebao. 2004;l7:71-72. |
| 15. | Holst MC, Powley TL. Cuprolinic blue (quinolinic phthalocyanine) counterstaining of enteric neurons for peroxidase immunocytochemistry. J Neurosci Methods. 1995;62:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981;75:816-821. [PubMed] |
| 17. | Asamoto K, Tamamaki N, Nojyo Y. Distribution of preganglionic terminals in the cervical sympathetic ganglia detected by the expression of c-Fos like protein after electric stimulation of the ventral root. Kaibogaku Zasshi. 2001;76:303-311. [PubMed] |
| 18. | Classey JD, Knight YE, Goadsby PJ. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity within the trigeminocervical complex following superior sagittal sinus stimulation in the cat. Brain Res. 2001;907:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | King VM, Apps R. Somatotopical organization of fos-like immunoreactivity in rat cervical spinal cord following noxious stimulation of the forelimb. Neuroscience. 2000;101:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Liu WG, Song JR, Lin JJ. Effect of bFGF on c-fos mRNA expre ssion in rats after spinal cord injury. Orthop J Chin. 2000;7:1178-1180. |
| 21. | Sugimoto T, Hara T, Shirai H, Abe T, Ichikawa H, Sato T. c-fos induction in the subnucleus caudalis following noxious mechanical stimulation of the oral mucous membrane. Exp Neurol. 1994;129:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Li T, Gao W, Rao ZR. Noxious somatic stimulation-induced expression of Fos-like immunoreactivity in catecholaminergic neurons with habenular nucleus projection in the medullary visceral zone of rat. Brain Res. 1998;783:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Ueyama T, Saika M, Senba E. Distinct gene expression in the stomach following stress and alcohol exposure. Kaibogaku Zasshi. 2001;76:435-441. [PubMed] |
| 24. | Rüter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L, Klapp BF, Wiedenmann B, Taché Y, Mönnikes H. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res. 2003;991:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Tada H, Fujita M, Harris M, Tatewaki M, Nakagawa K, Yamamura T, Pappas TN, Takahashi T. Neural mechanism of acupuncture-induced gastric relaxations in rats. Dig Dis Sci. 2003;48:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Schicho R, Schemann M, Pabst MA, Holzer P, Lippe IT. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol Motil. 2003;15:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Berthoud HR, Patterson LM, Zheng H. Vagal-enteric interface: vagal activation-induced expression of c-Fos and p-CREB in neurons of the upper gastrointestinal tract and pancreas. Anat Rec. 2001;262:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Emond M, Schwartz GJ, Moran TH. Meal-related stimuli differentially induce c-Fos activation in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1315-R1321. [PubMed] |
| 29. | Li GZ, Han DR, Chu CP, Cui SY. Gastrointestinal tract noxious stimulation induced fosexpression in nucleus of solitary tract, supraoptic nucleus and paraventricular nucleus in rat. Jieboxue Zazhi. 1998;21:317-320. |
| 30. | Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain. 2002;95:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889:118-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Sharkey KA, Kroese AB. Consequences of intestinal inflammation on the enteric nervous system: neuronal activation induced by inflammatory mediators. Anat Rec. 2001;262:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Luo JY, Niu CY. New conception of functional gastrointestinal disorders and disorders of gastrointestinal motility. Chin J Dig. 2002;22:554-558. |
| 34. | Dai F, Gong J, Zhang R, Luo JY, Zhu YL, Wang XQ. Assessment of duodenogastric reflux by combined continuous intragastric pH and bilirubin monitoring. World J Gastroenterol. 2002;8:382-384. [PubMed] |
| 35. | Shi Q. Attentional study in cervical spondylosis. Chin J Trad Med Traum Orthop. 1999;7:1-3. |
| 36. | Jiang GL, Li JJ, Xia XY, Xiao L. A clinical observation on the treatment of neck-stomach syndrome by three-step acupuncture and cupping. Xinzhongyi. 2002;34:49-50. |
| 37. | Blackshaw LA, Smid SD, O'Donnell TA, Dent J. GABA(B) receptor-mediated effects on vagal pathways to the lower oesophageal sphincter and heart. Br J Pharmacol. 2000;130:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Zhou L. Physiology and pathophysiology in the pathogenesis of Functional gastrointestinal motility disorders. Zhongguo Shiyong Neike Zazhi. 2001;21:577-579. |
| 39. | Kummer W, Gibbins IL, Stefan P, Kapoor V. Catecholamines and catecholamine-synthesizing enzymes in guinea-pig sensory ganglia. Cell Tissue Res. 1990;261:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Utzschneider D, Kocsis J, Devor M. Mutual excitation among dorsal root ganglion neurons in the rat. Neurosci Lett. 1992;146:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |