Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.511
Revised: February 6, 2004
Accepted: March 16, 2004
Published online: January 28, 2005
AIM: Cardiotonic Pill (CP), an oral herbal medicine that includes Danshen (Salviae Miltiorrhizae), Panax notoginseny and Dyroblanops aromatica gaertn, has been clinically used for vascular diseases such as occlusive vasculitis, coronary diseases, atherosclerosis, and cerebral infarction. The main component, Salviae Miltiorrhizae, has been reported to prevent cerebral and intestinal reperfusion injury. However, little is known about the effect of CP on hepatic microcirculation. Thus, this study aimed to determine whether CP could affect hepatic microvascular dysfunction elicited by gut ischemia/reperfusion (I/R) in rats fed ethanol chronically.
METHODS: Male Wistar rats were pair-fed with a liquid diet containing ethanol or isocaloric control diet for 6 wk. After laparotomy, one lobe of the liver was examined through an inverted intravital microscope. The rats were exposed to 30 min of gut ischemia followed by 60 min of reperfusion. Rhodamine-6G-labeled leukocytes in the sinusoids were observed 90 min after the onset of superior mesenteric artery occlusion. Plasma tumor necrosis factor (TNF)-α and endotoxin levels were measured 1 h after the onset of reperfusion. Plasma alanine aminotransferase (ALT) activities were measured 6 h after the onset of reperfusion. In another set of experiments, CP (0.8 g/kg, intragastrically) was administered 1 and 24 h before the onset of ischemia.
RESULTS: In control rats, gut I/R elicited increases in the number of stationary leukocytes, and plasma TNF-α and endotoxin levels and plasma ALT activities. These changes were mitigated by pretreatment with CP. In ethanol-fed rats, the gut I/R-induced increases in the number of stationary leukocytes, plasma endotoxin levels and ALT activities were enhanced. Pretreatment with CP attenuated the enhancement of gut I/R-induced responses by chronic ethanol consumption.
CONCLUSION: These results suggest that CP prevents the gut I/R-induced hepatic microvascular dysfunction and hepatocellular injury. A reduction of inflammatory responses such as TNF-α production via reduction of blood endotoxin levels appears to be involved in the mechanisms. Chronic ethanol consumption enhances gut I/R-induced hepatic microvascular and hepatocellular injury. CP also attenuates an enhancement of gut I/R-induced responses by chronic ethanol consumption via the reduction of blood endotoxin levels.
- Citation: Horie Y, Han JY, Mori S, Konishi M, Kajihara M, Kaneko T, Yamagishi Y, Kato S, Ishii H, Hibi T. Herbal cardiotonic pills prevent gut ischemia/reperfusion-induced hepatic microvascular dysfunction in rats fed ethanol chronically. World J Gastroenterol 2005; 11(4): 511-515
- URL: https://www.wjgnet.com/1007-9327/full/v11/i4/511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.511
A large body of evidence implicates leukocytes as mediators of microvascular dysfunction and tissue injury associated with reperfusion of ischemic organs. Several experimental strategies have been used to demonstrate the contribution of leukocytes to ischemia/reperfusion (I/R) injury, including polyclonal antibodies that render animal leukopenia[1-3], adhesion molecule-specific monoclonal antibodies[1,4-6], and adhesion molecule deficiency in mice[7,8]. The effectiveness of adhesion molecule-specific monoclonal antibodies (MAbs) and adhesion molecule-deficiency in attenuating I/R-induced tissue injury have led to the widely held view that leukocyte-endothelial cell adhesion is a rate-determining step in the pathogenesis of this injury process. We developed a leukocyte-dependent model of hepatocellular dysfunction elicited by gut I/R[1,7,9]. This murine model allows in vivo assessment of the effects of I/R on leukocyte sequestration in sinusoids of different regions of the liver lobule, leukocyte adherence in postsinusoidal venules, and the number of perfused sinusoids.
Ethanol has also been reported to modulate I/R-induced tissue injury[10-12]. In a perfused liver model, ethanol enhanced I/R-induced hepatotoxicity (an increase in blood levels of liver enzymes) by an enhanced production of reactive oxygen species[10]. In an in vivo gut I/R model, ethanol also enhanced gut I/R-induced neutrophil accumulation in the intestinal wall[11]. In an in vivo cerebral I/R model, however, ethanol pretreatment was reported to reduce cerebral I/R injury[12]. We have recently reported that low-dose ethanol attenuates gut I/R-induced hepatic microvascular dysfunction in the midzonal region and sequential hepatocellular injury, whereas high-dose ethanol enhances hepatic microcirculatory disturbances in the pericentral region and sequential hepatocellular injury[13].
Clinically, long-term alcohol consumption has been noted to significantly reduce the incidence of coronary artery disease[14]. However, chronic alcohol consumption often results in fatty liver and liver failure, which is of particular concern when such fat-laden tissues are used as donor organs in liver transplantation. This important clinical problem has drawn attention to the relationship between ethanol consumption and reperfusion injury in the liver. Both gut I/R and chronic consumption of ethanol are known to cause liver injury via mechanisms that involve oxidative stress and microcirculatory disturbances that include leukocyte sequestration and sinusoidal malperfusion[15]. Gut I/R is known to produce an elevation in plasma endotoxin levels (9), while chronic ethanol consumption has been reported to enhance the hepatic microcirculatory dysfunction and hepatocellular injury induced by endotoxins[16-18]. Based on these observations one might expect that chronic ethanol consumption would lead to an exaggerated liver injury response to gut I/R. Furthermore, we have recently reported that chronic ethanol consumption enhances hepatic leukosequestration, impaired sinusoidal perfusion, and hepatocellular injury caused by gut I/R via expression of intercellular adhesion molecule (ICAM)-1[19].
Herbal medicines (traditional medicines from natural sources) are recently attracting increased global attention. Further, Chinese herbal medicines have stood the test of time and many are widely accepted as having reliable therapeutic efficacy. Cardiotonic Pill (CP), an oral herbal medicine that includes Danshen (Salviae Miltiorrhizae), Panax notoginseny and Dyroblanops aromatica gaertn, has been clinically used in China, Korea and Russia for vascular diseases such as occlusive vasculitis, coronary diseases, atherosclerosis, and cerebral infarction, which are related to microvascular dysfunction. In clinical cases, the main component, Danshen, has been reported to have curative efficacy in ischemic cerebrovascular disease. In animal models, Danshen has been reported to prevent cerebral and intestinal reperfusion injury[20,21]. In the rat cerebral ischemia model, Danshen was reported to improve cerebral blood flow in the ischemic hemisphere and to inhibit platelet aggregation in rats[20]. However, little is known about the effect of CP on hepatic microcirculation. Hence, the overall objectives of this study was to assess the effects of CP on hepatic microvascular dysfunction and hepatocellular injury induced by gut I/R, and to determine whether CP could affect the hepatic microvascular dysfunction elicited by gut I/R in rats fed ethanol chronically.
Thirty (15 pairs) male Wistar rats weighing about 150 g were pair-fed for 6 wk with a liquid diet containing ethanol that provided 36% of the total dietary calories or an isocaloric control diet according to the method of Lieber et al[22]. All rats were fasted for18 h prior to the experiments, which were all performed according to the criteria outlined in the US National Research Council and the Keio Animal Research Guides.
Rats were anesthetized with pentobarbital sodium (35 mg/kg) intraperitoneally. The left carotid artery was cannulated and a catheter placed at the aortic arch to monitor blood pressure. The left jugular vein was also cannulated for drug administration. After laparotomy, a lobe of the liver was observed with an inverted intravital microscope (TMD-2S, Diaphoto, Nikon, Tokyo, Japan) assisted by a silicon intensified target (SIT) camera (C-2400-08, Hamamatsu Photonicus, Shizuoka, Japan). The liver was placed on an adjustable Plexiglas microscope stage with a non-fluorescent coverslip that allowed for observation of a 2-cm² segment of tissue. The liver was carefully placed to minimize the influence of respiratory movements, and the surface was moistened and covered with cotton gauze soaked with saline. Images of the microcirculation were observed from the surface of the liver through a ×20 fluorescent objective, and microfluorographs were recorded on videotape using a videocassette recorder (S VHS-HQ, Victor, Japan).
Leukocytes were labeled in vivo with rhodamine-6G (1 mg was dissolved in 5 mL of 0.9% saline) using a previously described method[12,17,19,23], which was based on a method used in rat brain[3]. It was shown that rhodamine 6G could selectively stain white blood cells and platelets, but not endothelial cells[3]. Thus, the fluorochrome allowed for differentiation between adherent leukocytes and endothelial cells. Rhodamine-6G (0.2 mL/100 g body weight) was injected prior to ethanol administration with subsequent injections every 30 min. Rhodamine-6G associated fluorescence was visualized by epi-illumination at 510-560 nm, using a 590 nm emission filter. We selected one of the lobules, which had well-perfused sinusoids and the fewest stationary leukocytes, choosing the furthest lobule from the edge of the liver if all the conditions were thought to be equivalent. A microfluorograph of hepatic microcirculation, with rhodamine-6G-labeled leukocytes in the sinusoids, was continuously observed for 90 min after the SMA occlusion and recorded on a digital video recorder for 1 min at 0, 30, 60, and 90 min. The number of stationary leukocytes was determined off-line during playback of the videotape images. A leukocyte was considered stationary within the microcirculation (sinusoids) if it remained stationary for more than 10 s. The lobule, which had the fewest stationary leukocytes was selected for observation at a basal condition. Stationary leukocytes were quantified in both the midzonal and pericentral regions of the liver lobule and expressed as the number per field of view (2.1×105µm2).
We observed the surface of the liver for 10 min before ligation of the superior mesenteric artery in order to ensure that all parameters measured on-line were in a steady state. The superior mesenteric artery was then ligated with a snare created from polyethylene tubing for 0 (sham) or 30 min. After the ischemic period, the ligation was gently removed. Leukocyte accumulation was measured before ischemia, immediately following reperfusion and every 30 min for one hour thereafter.
In another set of experiments, CP (0.8 g/kg, intragastrically, Tasley, Tianjin, China) was administered at 1 and 24 h before the onset of ischemia to both control and ethanol-fed rats, and the same protocol was performed. Twenty-five mg (one pill) of CP includes 9 mg of Danshen (Salviae Miltiorrhizae), 1.76 mg of Panax notoginseny, 0.5 mg of Dyroblanops aromatica gaertn, and 13.74 mg of polyethylene glycol.
Sixty min after the onset of reperfusion, the rats were removed from the microscope stage and the abdominal wall was closed. Blood (plasma) samples for endotoxin and tumor necrosis factor (TNF)-α level measurement were collected from the inferior vena cava at a point proximal to the hepatic vein 1 h after the onset of reperfusion. For the measurement of endotoxin levels, blood samples were also collected from the portal vein. Samples for plasma alanine aminotransferase (ALT) measurement were obtained 6 h after the onset of reperfusion. Plasma ALT activity was determined by conventional UV method as previously described[24]. Plasma TNF-α concentration was determined in a microtiter plate using a TNF-α immunoassay kit (BioSource International, Camarillo, CA) based on enzyme-linked immuno sorbent assay (ELISA). In accord with our previous report[25,26], plasma endotoxin levels were measured by endospecy (an endotoxin-specific chromogeic limulus regent; Seikagaku Co., Tokyo, Japan) using an automated kinetic assay for endotoxin[27].
The data were analysed using standard statistical analyses, i.e., ANOVA and Scheffe’s (post hoc) test. All values were reported as mean±SD, with five rats per group. Statistical significance was set at P<0.05.
Table 1 shows the effects of CP treatment on leukostasis in sinusoids of the midzonal and pericentral regions and the terminal hepatic venule (THV), plasma ALT activities, and plasma endotoxin and TNF-α levels. Pretreatment with CP per se did not affect any values of these in control rats. Chronic ethanol feeding per se caused leukostasis in the pericentral region of the liver, while CP diminished it.
| Group | Leukostasismidzonal (per field) | Pericentral (per field) | THV (per field) | ALT activity(IU/L) | Endotoxin(pg/mL) | TNF-a (pg/mL) |
| Control | 1.8±0.7 | 1.0±0.7 | 0.5±0.5 | 31.6±12.0 | 9.5±5.3 | 17.0±8.9 |
| Ethanol | 1.4±0.5 | 3.2±0.5a | 1.2±0.7 | 40.6±10.5 | 19.2±10.8 | 23.1±15.1 |
| Control+CP | 1.4±1.2 | 1.0±1.0 | 0.2±0.5 | 28.4±11.8 | 9.3±5.3 | 13.3±10.1 |
| Ethanol+CP | 1.6±0.5 | 0.8±0.5 | 0.6±1.0 | 36.6±7.2 | 13.6±5.5 | 10.6±6.2 |
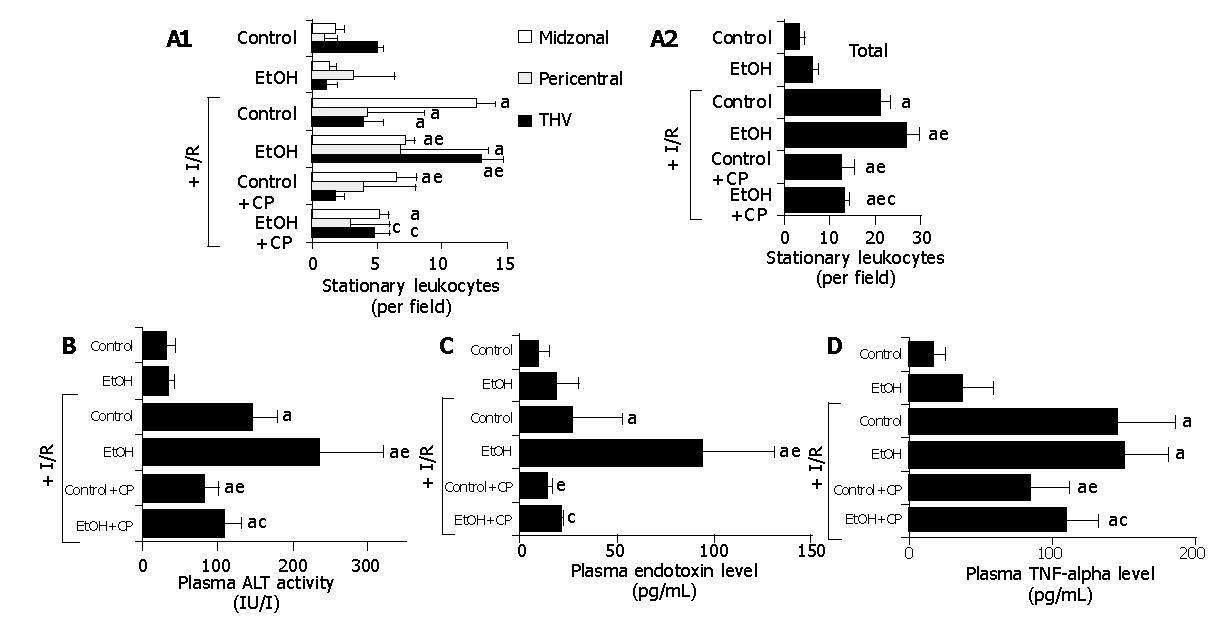
Figure 1A illustrates the effects of CP treatment on gut I/R-induced leukostasis in sinusoids of the midzonal and pericentral regions and the THV (Panel A) of the liver lobule, and the entire liver lobule (sinusoids+THV, panel B) in the presence or absence of chronic ethanol consumption In control rats, gut I/R elicited increases in the number of stationary leukocytes in both hepatic sinusoids and THV. In ethanol-fed rats, the gut I/R-induced leukostasis was blunted in the midzonal region (control; 12.6±1.4, ethanol: 7.2±0.7, per field), while exaggerated leukostasis was noted in the pericentral region (control; 4.3±1.9, ethanol: 6.8±2.2) and THV (control; 4.0±1.4, ethanol: 13.0±1.6, per field). Although the leukostasis elicited by gut I/R in control rats was attenuated by the pretreatment with CP (leukostasis in the pericentral region; 4.0±1.2, THV; 1.8±0.7, per field), the exaggerated leukostasis in ethanol-fed rats was largely prevented by pretreatment with CP (leukostasis in the pericentral region; 3.0±0.7, THV; 4.8±1.2, per field).
Figure 1B shows the effects of CP treatment on plasma ALT activity following gut I/R in the presence or absence of chronic ethanol consumption. In control rats, gut I/R led to an elevation of plasma ALT activities. Chronic ethanol consumption enhanced the gut I/R-induced increase in plasma ALT activities (control; 146±31 IU/L, ethanol: 236±84 IU/L). The increase in plasma ALT activities elicited by gut I/R in both control and ethanol-fed rats was significantly attenuated by pretreatment with CP (control; 81±19 IU/L, ethanol: 108±58 IU/L).
Figure 1C shows the effects of CP treatment on plasma endotoxin levels following gut I/R in the presence or absence of chronic ethanol consumption. Gut I/R caused a slight elevation of plasma systemic and portal endotoxin levels in control rats, while chronic ethanol consumption enhanced the gut I/R-induced increase in plasma endotoxin levels (control; 26.2± 27.1 pg/mL, ethanol: 93.2±51.4 pg/mL). The exaggerated elevation of plasma endotoxin levels in ethanol-fed rats was largely prevented by the pretreatment with CP (endotoxin levels; 21.3± 1.4 pg/mL).
Figure 1D summarizes the effects of CP treatment on gut I/R-induced increase in plasma TNF-α levels in the presence or absence of chronic ethanol consumption. In control rats, gut I/R elicited a significant increase in plasma TNF-α levels. Although chronic ethanol consumption did not affect gut I/R-induced increases in plasma TNF-α levels, CP treatment reduced plasma TNF-α in both control and ethanol-fed rats.
Our previous study[19] has demonstrated that leukocyte-endothelial cell adhesion is an important determinant of the exaggerated microvascular dysfunction and tissue injury after gut I/R in the liver of rats fed ethanol chronically. The main component of CP is Danshen. Seven water-soluble components have been isolated from the Danshen root, Radix Salviae Miltiorrhizae. Using HPLC, at least 10 peaks were resolved based on its affinity to α 1-acid glycoprotein[28]. One of the components, salvianolic acid, was reported to protect cerebral I/R injury in rats[20]. Althogh CP had a protective effect on carbon tetrachloride-induced hepatocellular injury[29], the effect of CP on either hepatic I/R injury or hepatic microcirculation has not been reported. In this study, we demonstrated the protective effects of CP on gut I/R-induced liver injury in rats fed ethanol chronically.
Reperfusion of the ischemic intestine in control rats resulted in an accumulation of adherent leukocytes in sinusoids and THV, a reduction in the number of perfused sinusoids, and the release of liver enzyme (ALT) into the blood stream. In control rats, gut I/R-induced leukostasis in the pericentral region and THV were not noted after pretreatment with CP. This pretreatment also attenuated the gut I/R-induced increase in plasma ALT and TNF-α levels. An interesting finding in the present study is that the gut I/R-induced increase in plasma endotoxin level was not seen in control rats after the pretreatment with CP. Gut I/R was reported to elevate plasma endotoxin levels, which appeared to be derived from the gut[19]. CP has been reported to blunt mesenteric I/R injury[21]. Endotoxin has been known to cause hepatic microvascular dysfunction and hepatocellular injury[17,18,24]. Taken together, these results and evidence from the literature suggest that CP can reduce blood endotoxin levels by protecting the intestinal mucosal barrier from I/R injury, thereby preventing the subsequent hepatic microvascular dysfunction and hepatocellular injury.
Chronic ethanol consumption exaggerated gut I/R-induced leukostasis in the liver, gut I/R-induced increase in plasma endotoxin levels, and the subsequent hepatocellular injury (ALT elevation). The findings in the present study lend support to the possibility that elevated plasma levels of endotoxin contribute to the exaggerated inflammatory and tissue injury responses seen in the liver after gut I/R in rats chronically fed ethanol. The portal endotoxin level in rats fed ethanol chronically was higher than that in controls[19]. This result suggested that intestinal mucosal permeability increased in ethanol-fed rats after gut I/R. However, systemic endotoxin levels were much lower than portal endotoxin levels in control rats, whereas there was no significant difference between systemic and portal endotoxin levels in ethanol-fed rats after gut I/R[19], suggesting that clearance of endotoxin was impaired in ethanol-fed rats. Thus, both an increase in intestinal mucosal permeability and impaired clearance of endotoxin in ethanol-fed rats could be involved in the enhancement of plasma endotoxin levels. In the present study, pretreatment with CP substantially reduced the exaggerated increase in plasma endotoxin levels in ethanol-fed rats to almost the same level as that in untreated rats (without I/R) This result suggests that CP might blunt the enhanced increase in intestinal mucosal permeability and /or improvised the impaired clearance of endotoxin in ethanol-fed rats.
Although chronic ethanol consumption enhanced the gut I/R-induced increase in plasma endotoxin levels, it did not affect the gut I/R-induced increase in plasma TNF-α levels. Chronic ethanol consumption enhanced the gut I/R-induced increase in plasma ALT activities with a parallel increase in leukostasis in the liver. These results suggest that leukostasis per se or leukocyte-derived oxidants may play a more important role in gut I/R-induced liver (hepatocellular) injury than cytokines. Another likely interpretation is that cytokines other than TNF-α are involved in the enhanced responses after gut I/R in rats fed ethanol chronically. In the present study, however, CP decreased the gut I/R-induced increase in plasma ALT activities with a parallel decrease in plasma TNF-α levels. Moreover, CP also attenuated the enhancement of gut I/R-induced increase in plasma ALT activities in ethanol-fed rats with a parallel attenuation of plasma TNF-α levels. These results indicate that CP can reduce the production of TNF-α independent of endotoxin levels. CP was also reported to blunt TNF-α-induced endothelial cell injury via NF-κB activation[30]. Therefore, CP may prevent gut I/R-induced hepatic microvascular dysfunction via protection of endothelial cells.
Thus, CP can protect gut I/R-induced hepatic microvascular dysfunction and hepatocellular injury. Although further studies and clinical trials are required, CP appears to have therapeutic usefulness against reperfusion injury in the liver.
Co-correspondents: Jing-Yan Han
Edited by Wang XL
| 1. | Horie Y, Wolf R, Miyasaka M, Anderson DC, Granger DN. Leukocyte adhesion and hepatic microvascular responses to intestinal ischemia/reperfusion in rats. Gastroenterology. 1996;111:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355-3359. [PubMed] |
| 3. | Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 837] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368-374. [PubMed] |
| 5. | Kurose I, Anderson DC, Miyasaka M, Tamatani T, Paulson JC, Todd RF, Rusche JR, Granger DN. Molecular determinants of reperfusion-induced leukocyte adhesion and vascular protein leakage. Circ Res. 1994;74:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 213] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Simpson PJ, Todd RF, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988;81:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 487] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Horie Y, Wolf R, Anderson DC, Granger DN. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest. 1997;99:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Kelly KJ, Williams WW, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 577] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 9. | Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia-reperfusion. Pathophysiology. 2001;8:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Younes M, Wagner H, Strubelt O. Enhancement of acute ethanol hepatotoxicity under conditions of low oxygen supply and ischemia/reperfusion. The role of oxygen radicals. Biochem Pharmacol. 1989;38:3573-3581. [PubMed] |
| 11. | Tabata T, Meyer AA. Ethanol ingestion potentiates PMN migration into small intestine after ischemia. J Surg Res. 1995;58:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Phillis JW, Estevez AY, O'Regan MH. Protective effects of the free radical scavengers, dimethyl sulfoxide and ethanol, in cerebral ischemia in gerbils. Neurosci Lett. 1998;244:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Yamagishi Y, Horie Y, Kato S, Kajihara M, Tamai H, Granger DN, Ishii H. Ethanol modulates gut ischemia/reperfusion-induced liver injury in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G640-G646. [PubMed] |
| 14. | Miyamae M, Diamond I, Weiner MW, Camacho SA, Figueredo VM. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury. Proc Natl Acad Sci USA. 1997;94:3235-3239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Ishii H, Kurose I, Kato S. Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J Gastroenterol Hepatol. 1997;12:S272-S282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Arai M, Nakano S, Okuno F, Hirano Y, Sujita K, Kobayashi T, Ishii H, Tsuchiya M. Endotoxin-induced hypercoagulability: a possible aggravating factor of alcoholic liver disease. Hepatology. 1989;9:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Horie Y, Kimura H, Kato S, Ohki E, Tamai H, Yamagishi Y, Ishii H. Role of nitric oxide in endotoxin-induced hepatic microvascular dysfunction in rats chronically fed ethanol. Alcohol Clin Exp Res. 2000;24:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Ohki E, Kato S, Ohgo H, Mizukami T, Fukuda M, Tamai H, Okamura Y, Matsumoto M, Suzuki H, Yokoyama H. Effect of chronic ethanol feeding on endotoxin-induced hepatic injury: role of adhesion molecules on leukocytes and hepatic sinusoid. Alcohol Clin Exp Res. 1998;22:129S-132S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Horie Y, Yamagishi Y, Kato S, Kajihara M, Tamai H, Granger DN, Ishii H. Role of ICAM-1 in chronic ethanol consumption-enhanced liver injury after gut ischemia-reperfusion in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G537-G543. [PubMed] |
| 20. | Tang MK, Ren DC, Zhang JT, Du GH. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine. 2002;9:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Han JY, Akiba Y, Suzuki H, Nagata N, Miura S, Oda M, Ishii H. Cardiotonic pills inhibits leukocyte adhesion induced by ischemia-reperfusion in rat mesenteric microcirculation. Microcirculation Annual Vol. 18 (eds. Asano M, and Ohkubo C.) Nihon Igakukan Tokyo 2002: 31-32. . |
| 22. | Lieber CS, Decarli LM. Animal models of ethanol dependence and liver injury in rats and baboons. Fed Proc. 1976;35:1232-1236. [PubMed] |
| 23. | Horie Y, Kato S, Ohki E, Tamai H, Ishii H. Role of endothelin in endotoxin-induced hepatic microvascular dysfunction in rats fed chronically with ethanol. J Gastroenterol Hepatol. 2001;16:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Horie Y, Kato S, Ohki E, Hamamatsu H, Fukumura D, Kurose I, Suzuki H, Suematsu M, Miura S, Ishii H. Effect of lipopolysaccharides on erythrocyte flow velocity in rat liver. J Gastroenterol. 1997;32:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res. 2000;24:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Tamai H, Horie Y, Kato S, Yokoyama H, Ishii H. Long-term ethanol feeding enhances susceptibility of the liver to orally administered lipopolysaccharides in rats. Alcohol Clin Exp Res. 2002;26:75S-80S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Tamura H, Arimoto Y, Tanaka S, Yoshida M, Obayashi T, Kawai T. Automated kinetic assay for endotoxin and (1--& gt; 3)-beta-D-glucan in human blood. Clin Chim Acta. 1994;226:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Wang H, Zou H, Kong L, Ni J. Analysis of bioactive components in traditional Chinese medicines by molecular biochromatography with alpha1-acid glycoprotein stationary phase. J Basic Clin Physiol Pharmacol. 2000;11:155-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Xie F, Li X, Sun K, Chu Y, Cao H, Chen N, Wang W, Liu M, Liu W, Mao D. An experimental study on drugs for improving blood circulation and removing blood stasis in treating mild chronic hepatic damage. J Tradit Chin Med. 2001;21:225-231. [PubMed] |
| 30. | Zhou-Stache J, Buettner R, Artmann G, Mittermayer C, Bosserhoff AK. Inhibition of TNF-alpha induced cell death in human umbilical vein endothelial cells and Jurkat cells by protocatechuic acid. Med Biol Eng Comput. 2002;40:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |