Published online Oct 21, 2005. doi: 10.3748/wjg.v11.i39.6221
Revised: May 9, 2005
Accepted: May 12, 2005
Published online: October 21, 2005
We present the fourth case of a primary pancreatic anaplastic large cell lymphoma (ALCL), ALK-. An 80-year-old man was admitted to our clinic for further investigation of a fever of unknown origin. He noted anorexia, weight loss and fatigue. His laboratory tests showed anemia and a great elevation of ESR, LDH, and β2 microglobulin. In CT and MRI scan, a soft tissue mass in the pancreas was observed. A repeated endoscopy after his admission revealed an ulcerated mass-like deformity of the duodenal bulb. Explorative laparotomy confirmed a diffuse spread of an unresectable malignant pancreatic mass extending to the adjacent organs. Duodenal and surgical biopsies identified an ALCL of T-cell lineage, ALK-. The patient died in the Intensive Care Unit due to hemodynamic instability. Our case is the first one indicating that primary pancreatic lymphoma should be suspected in a patient with pancreatic mass and elevated serum LDH and β2 microglobulin.
- Citation: Savopoulos CG, Tsesmeli N, Kaiafa G, Zantidis A, Bobos M, Hatzitolios A, Papavramidis S, Kostopoulos I. Primary pancreatic anaplastic large cell lymphoma, ALK negative: A case report. World J Gastroenterol 2005; 11(39): 6221-6224
- URL: https://www.wjgnet.com/1007-9327/full/v11/i39/6221.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i39.6221
Malignant tumors of the pancreas constitute about 15% of cancer patients and the most frequent of them is the adenocarcinoma of the pancreas[1]. Primary pancreatic lymphoma (PPL) is a very rare disease constituting less than 0.5% of all pancreatic malignancies and less than 2% of extranodal lymphomas[2]. Primary pancreatic anaplastic large cell lymphomas (PPALCL) are extremely rare. Only three cases of PPALCL have been reported in the literature[1,3,4]. Herein we report the fourth case of PPALCL which was diagnosed by duodenal and surgical biopsies.
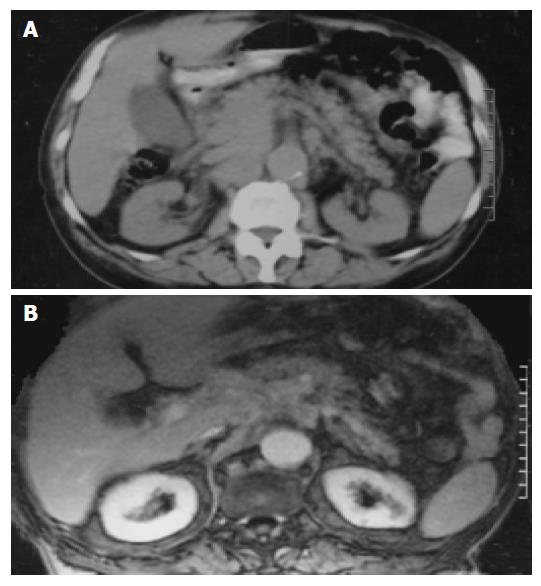
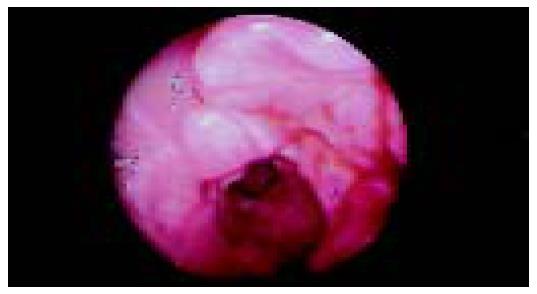
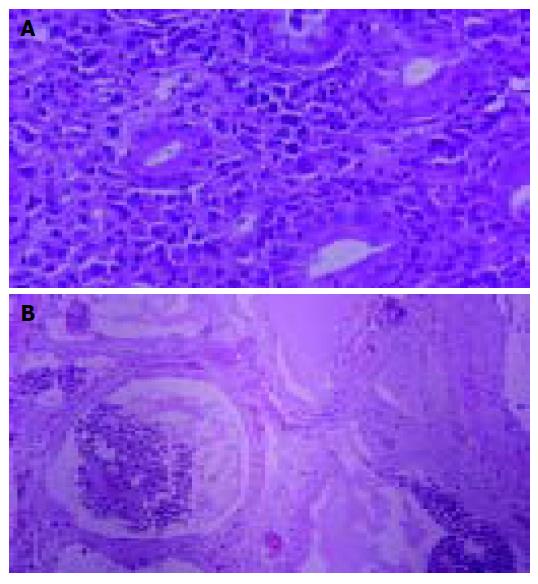
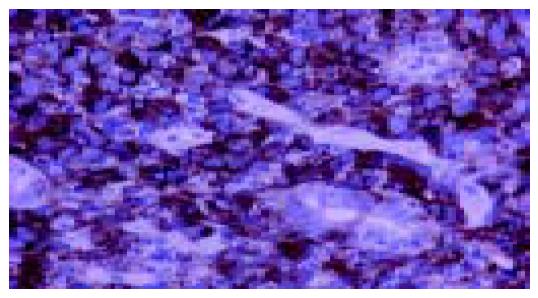
An 80-year-old man, who was under evaluation of a fever of unknown origin in a Private Health Center, was admitted to our clinic for further investigation. He was complaining of an intermittent fever of 2 mo duration which stabilized during the last 3 wk. Anorexia, weight loss of about 10 kg in the last 6 mo and fatigue were also reported. Physical examination was unremarkable except for a leg edema. His hematological profile was as follows: Hct: 31%, Hb: 9.9 g/L, MCV: 92 fl, MCH: 30 pg/cell, reticulocytes: 1.6%, WBC: 10.6?09 cells/L (neutrophils 80% -lymphocytes 11% -monocytes 6% -eosinophils 2.5% -basophils 0.5%), PLT: 389?09/L, ESR: 110 mm, CRP: 13 mg/dL (normal range: 0-0.8 mg/dL). Serum biochemistry showed a great elevation of LDH: 1 880 U/L (normal range: 313-618 U/L) and hypoalbuminemia: 2.1 g/L (normal range: 3.4-4.8 g/L), with normal total proteins: 7.1 g/L (normal range: 6.6-8.7 g/L) though. Elevated β2 microglobulin: 4.19 mg/L (normal range: 1.42-3.21 mg/L) was also found. Other biochemical, serologic and immunologic investigations were normal. Laboratory studies performed after the onset of the symptoms, with values similar to our findings, were available from the patient's file. A chest and abdominal CT scan, also available to us, had demonstrated that the uncinate process and partially the head of the pancreas were blurred (Figure 1A); enlarged peripancreatic, mesenteric, para-aortic and inferior vena cava lymph nodes were found. At that time, he had undergone a gastrointesinal endoscopy without pathologic findings. In further imaging study with MRI scan, a soft tissue mass located in the head of the pancreas and especially the uncinate process had appeared (Figure 1B). A slow flow signal of inferior vena cava had been observed due to the mass compression. After his admission in our clinic, a new endoscopy of the upper gastrointestinal tract revealed an ulcerated mass-like deformity of the duodenal bulb with a friable surface (Figure 2). Gastric and duodenal mucosal biopsy specimens were taken. Bone marrow biopsy was also performed. Glucocorticoids were administrated as a second choice therapy after antibiotics treatment, based on FUO treatment's protocol and on the high indication of a lympho-proliferative disorder. Temporary clinical and laboratory improvement was noticed (ESR declination: 55 mm). Explorative laparotomy confirmed a diffuse spread of a malignant mass of the pancreas extending to the adjacent organs. Because of the brittle consistency and necrotic surface of the pancreatic mass, the biopsy specimen was taken from the adhesion of pancreatic surface with gastric serosa. Hematoxylin-and-eosin-stained sections of the duodenal biopsy revealed a diffuse dense infiltration of duodenal mucosa by large pleomorphic cells with abundant eosinophilic cytoplasm, ovoidal or irregular nuclei with one or prominent nucleoli (Figure 3A). Rare cells with embryo-like, horseshoe-shaped nuclei or cells with multiple nuclei resembling Reed-Sternberg cells were found. The mitotic activity was intermediate and several atypical mitoses were observed. Scanty neoplastic cells infiltrated the epithelium of intestinal glands as well as the surface epithelium. Histologic sections from the second biopsy material showed loose connective tissue with numerous dilated vessels and lymphatics, most of them having tumor cell emboli with morphology similar to the neoplastic population of the duodenal mucosa (Figure 3B). The intravascular presence of neoplastic cells was confirmed by immunohistochemistry using the endothelial cell marker CD34 (Novocastra, Newcastle, UK). Immunohistochemical stains of both biopsies showed the following tumor-cell immunophenotype: CD45+, CD45RO+, EMA+, CD43+ (DakoCytomation, Glostrup, Denmark), CD30+ (Figure 4), Muc1+, Fascin+ (Novocastra, Newcastle, UK), CD20-, CD45RA-, CAM 5.2-, Anaplastic Lymphoma Kinase (ALK)1-(DakoCytomation, Glostrup, Denmark) and CD3- (Novocastra, Newcastle, UK). Cellular phenotype and immunophenotype support the diagnosis of anaplastic large cell lymphoma (ALCL), of T-cell lineage, ALK negative. Fluorescent in situ hybridization (FISH) using LSI ALK dual color, break apart rearrangement probe (Abbott GmbH and Company, KG, Wiesbaden-Delkenheim, Germany) was performed in paraffin-embedded tissue sections from both specimens. Presence of two fused yellow signals or two adjacent (one orange and one green signals) in 200 non-overlapping tumor-cell nuclei showed lack of ALK gene rearrangement at 2p23 region. Bone marrow biopsy showed no evidence of lymphoma involvement. The patient died in the Intensive Care Unit 2 d later due to hemodynamic instability.
PPL is an extremely rare neoplasm that may be confused with the most common pancreatic adenocarcinoma. The majority of PPL reported to date in literature have been classified as B-cell type, but several cases of T-cell pancreatic lymphomas have also been described[2]. Most cases are intermediate or high grade NHL with diffuse large B-cell lymphoma being the predominant type. Presenting symptoms are nonspecific, including abdominal pain, weight loss, nausea, vomiting. Systemic-B symptoms such as fever, chills and night sweats are uncommon. Imagining techniques such as CT, percutaneous U/S and recently endoscopic U/S are the most useful procedures to evaluate the staging of pancreatic masses, although they cannot identify their neoplastic nature. A cytohistological diagnosis can be performed by U/S and CT guided techniques, endoscopy and explorative laparotomy[2]. Chemotherapy treatment with CHOP (cyclophosphamide, adriamycin, vincristine, and prednisolone) is usually administrated in patients with lymphomas[1,2]. The accurate distinction of ALCL from non-lymphoid malignancies is critical from the standpoint of patient's management because primary ALCL responds to radically different chemotherapy than adenocarcinoma[5,6].
ALCL is an uncommon type of NHL, first described by Stein et al[7] in 1985 as a pleomorphic large cell lymphoma with strong membrane and Golgi associated CD30/Ki-1 antigen expression in a very high percentage of neoplastic lymphoid cells, with the involvement of paracortical region and sinuses of lymph nodes. In the latest World Health’s Organization (WHO) classification of NHLs, ALCL is a distinctive nature T-cell lymphoma subtype[5]. Clinical presentations of ALCL include a systemic form with nodal and/or extranodal involvement, primary cutaneous ALCL, HIV-related ALCL and ALCL occurring as a secondary event in patients with lymphomatoid papulosis, mycosis fungoides and rarely Hodgkin disease. The main histologic patterns accepted by WHO are the common variant, the lymphohistiocytic and the small cell variant[5]. In these lymphomas, the most frequent genetic alteration is the translocation t (2; 5) (p23; q35) between the ALK gene on chromosome 2 and the nucleophosmin (NPM) gene on chromosome 5. As a result of this translocation, the hybrid (NPM-ALK) gene promotes the production of chimeric NPM-ALK protein. The NPM-ALK fusion chimeric protein can be detected immunohistochemically using monoclonal or polyclonal antibodies, by RT-PCR and FISH[5,8]. ALK immunohistochemical expression is detected in 60-85% of ALCL cases[5]. ALK+ ALCL is most frequent in the first decades of life, shows a male predominance and often has advanced stage disease with frequent B-symptoms and extranodal involvement. It also tends to respond better to chemotherapy than ALK-systemic ALCL[5,8].
Our patient was considered to be affected by PPL according to Dawson et al[9] clinical criteria which include: (1) the absence of superficial lymphadenopathy or enlargement of mediastinal lymph nodes on chest radiography, (2) a normal leukocyte count in peripheral blood, (3) main mass in the pancreas with lymph-nodal involvement confined to the peripancreatic region, and (4) the absence of hepatic or splenic involvement. The elevated values of LDH and β2 microglobulin in addition to the clinical characteristics of our patient, such as intermittent fever, anorexia, weight loss and fatigue, also suggested the lymphoproliferative nature of the mass. Moreover, the presence of the mass in the pancreas at imaging techniques while the duodenum was normal in the first endoscopy, proved that it originated in the pancreas and not in the duodenum which is an extremely rare location also.
Only three patients with primary pancreatic ALCL are reported in the literature. The first case described in 1997 in Japan by Maruyama et al[3] was diagnosed by both duodenal and surgical pancreatic biopsies. The second case was published in 2003 in Israel by Cohen et al[1] and was diagnosed by operative pancreatic biopsy. Chim et al[4] recently reported the third case and they supported the diagnosis on operative mesenteric lymph node material biopsy because the tumor resection was not possible. In our case a pancreaticodu-odenectomy, the "Whipple procedure" was not performed, as in the case of Chim et al because of a friable tissue consistency and patient's hemodynamic instability during surgery. Therefore, the diagnosis of our PPALCL was based on the biopsy material taken from duodenal mucosa (three small pieces) and the adhesion of pancreatic surface with gastric serosa.
LDH and β2 microglobulin are considered to be tumor markers in lymphoproliferative disorders and have an important prognostic value[10,11]. According to the textbook of Sleisenger and Fordtran's, whenever a large mass is identified in the pancreas without biliary obstruction or pain the diagnosis of pancreatic lymphoma should be contemplated, particularly in the presence of an elevated serum LDH level[12]. A high LDH level was found in our patient in contrast to the three previously reported cases of PPALCL. The LDH values were normal in the cases of Chim et al and Cohen et al and non-recorded in the case of Maruyama et al In addition, an elevated β2 microglobulin level was noticed in our case such as reported in ALCL either ALK+ or ALK-in the study of Rassidakis et al[11].
After chemotherapy administration, a remission of disease for 30 mo in the case reported by Cohen et al and for 18 mo in the case reported by Maruyama et al were achieved respectively, whereas in the recently reported case by Chim et al a partial remission of 6 mo duration was noticed because the patient died of disease progression. Our patient did not receive a postoperative chemotherapy because unfortunately he died 2 d after the explorative laparotomy.
The clinical information, laboratory and pathologic findings of the present case and the previously reported ones, are summarized in Table 1.
| Maruyama et al[3] | Cohen et al[1] | Chim et al[4] | Present study | |
| A/G | 46/F | 22/M | 27/F | 80/M |
| Clinical symptoms | Back pain, anorexia | Upper abdominal pain, upper GI bleeding | Upper abdominal pain, weight loss | FUO, anorexia, weight loss, fatigue |
| Physical examination | Icteric conjunctivae, flat and soft abdomen with mild tenderness | Pallor, epigastric mass | Epigastric mass | Leg edema |
| Radiological findings | CT: mass in pancreatic head, ERP: without pathologic changes in MPD | CT: mass in pancreatic head | CT: mass in pancreatic head | CT: mass in pancreatic head and uncinate process, MRI: CT findings + IVC compression |
| Endoscopy | Submucosal protrusion with erosion of the second portion of the duodenum | Duodenal bleeding near to Vater’s papilla | Bleeding ulceration of the first portion of the duodenum | Ulcerated mass-like deformity of the duodenal bulb |
| Laboratory findings | ALT, AST, ALP, total and direct bilirubin elevated, serum amylase decreased, LDH: ND | Peripheral eosinophilia, LDH: in normal range | LDH: in normal range | LDH: elevated, β2 microglobulin: elevated |
| Surgical operation | Whipple’s | Whipple’s | Not performed | Not performed |
| Pathologic (IHC) findings | CD30+, CD45+, CD45RO+, EMA-, ALK: ND | CD30+, CD45RO+, CD3+, EMA+, ALK- | CD30+, CD45-, CD45RO+, ALK: ND | CD30+, CD45+, CD45RO+, EMA+, CD3-, ALK- |
| Treatment | MACOP-P | CHOP | CHOP | Glucocorticoids (symptomatic) |
| Follow up | NED at 18 months | NED at 30 months | 6 months, died of disease progression | Died due to haemodynamic instability 2 days after explorative laparotomy |
In conclusion, our case - added to the three published cases - is the fourth one of primary pancreatic ALCL, ALK-. From the clinical point of view, since most clinicians do not consider PPL in the differential diagnosis of a pancreatic mass, our case indicates that PPL should be suspected in a patient with pancreatic mass and elevated serum markers, particularly LDH and β2 microglobulin. Biopsy specimens?results from either the mass or the adjacent infiltrated organs, such as the duodenum in our case, confirm the diagnosis and identify the lymphoma. Thus, an earlier diagnosis leads to an appropriate and timeable treatment and finally, a better prognosis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Cohen Y, Libster D, Amir G, Hiller N, Da'as N, Ben Yehuda D, Polliack A. Primary ALK positive anaplastic large cell lymphoma of the pancreas. Leuk Lymphoma. 2003;44:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Arcari A, Anselmi E, Bernuzzi P, Bertè R, Lazzaro A, Moroni CF, Trabacchi E, Vallisa D, Vercelli A, Cavanna L. Primary pancreatic lymphoma. Report of five cases. Haematologica. 2005;90:ECR09. [PubMed] |
| 3. | Maruyama H, Nakatsuji N, Sugihara S, Atsumi M, Shimamoto K, Hayashi K, Tsutsumi M, Konishi Y. Anaplastic Ki-1-positive large cell lymphoma of the pancreas: a case report and review of the literature. Jpn J Clin Oncol. 1997;27:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Chim CS, Ho J, Ooi GC, Choy C, Liang R. Primary anaplastic large cell lymphoma of the pancreas. Leuk Lymphoma. 2005;46:457-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Delsol G, Ralfkiaer E, Stein H, Wright D, Jaffe ES. Anaplastic large cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Pathology & Genetics. Tumors of Haematopoetic and Lymphoid Tissues. Lyon IARC Press 2001; 230-235. |
| 6. | Yoshikawa I, Murata I, Tanaka Y, Kanagawa K, Tabaru A, Itoh H, Otsuki M. Case report: primary CD30 (Ki-1)-positive anaplastic large cell lymphoma of the duodenum. Dig Dis Sci. 1996;41:2343-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848-858. [PubMed] |
| 8. | Greer JP, Kinney MC, Loughran TP. T cell and NK cell lymphoproliferative disorders. Hematology Am Soc Hematol Educ Program. 2001;72:259-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 468] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Cooper D. Tumor Markers. In: Goldman L, Ausiello D, eds. Cecil Textbook of Medicine, 22nd edition. Philadelphia SAUNDERS 2004; 1131-1134. |
| 11. | Rassidakis GZ, Goy A, Medeiros LJ, Jiang Y, Thomaides A, Remache Y, Cabanillas F, Sarris AH, Gilles F. Prognostic significance of MUC-1 expression in systemic anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:2213-2220. [PubMed] |
| 12. | Fernandez-del Castillo C, Jimenez RE. Pancreatic cancer, cystic pancreatic neoplasms, and other nonendocrine pancreatic tumors. In: Sleisenger & Fordtran's, eds. Gastrointestinal and Liver Disease, 7th edition. Philadelphia SAUNDERS 2002; 970-987. |