Published online Oct 21, 2005. doi: 10.3748/wjg.v11.i39.6212
Revised: March 23, 2005
Accepted: March 21, 2005
Published online: October 21, 2005
AIM: To investigate the contribution of HBV in the development of hepatocarcinoma by examining the effects of HBV on p53 function in SMMU-7721 cell line.
METHODS: Plasmid pCMVp53 was transfected or cotransfected with pCMVHBVa (wild-type HBV) or PCMVHBVb (mutation type HBV) into the hepatoma cell line SMMU-7721 by lipofectamine. Apoptosis cells were labeled with annexin V-FITC and confirmed by flow cytometry. Reporter plasmid PG13-CAT or p21-luc was cotransfected, respectively, into each group to determine the transactivation activity of p53 and its effect on p21 promoter. Western blot was performed to observe p53 expression in hepatoma cell line of each group.
RESULTS: The group transfected with pCMVp53 alone exhibited higher luciferase activity and higher apoptosis rate, otherwise, the p53 expression and reporter activity of PG13-CAT or P21-luc as well as cell apoptosis rate were obviously higher in the group cotransfected of pCMVp53 with pCMVHBVa, but not in the other cotransfected group.
CONCLUSION: Transient transfection of HBV into the SMMU-7721 cell line can enhance p53 expression and its effects on development of hepatocarcinoma.
- Citation: Qu JH, Zhu MH, Lin J, Ni CR, Li FM, Zhu Z, Yu GZ. Effects of hepatitis B virus on p53 expression in hepatoma cell line SMMU-7721. World J Gastroenterol 2005; 11(39): 6212-6215
- URL: https://www.wjgnet.com/1007-9327/full/v11/i39/6212.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i39.6212
HBV is a major etiological factor associated with hepatocarcinogenesis, but its role in the transformation process remains unknown. p53 is an important tumor suppressor and its mutation or inactivation is thought to be involved in the development of various types of cancers, including primary hepatocellular carcinoma (PHC)[1]. It has been reported that the p53 protein could bind with HBx antigen to form the complexes, which led to p53 inactivation[2,3]. Genetic alterations in the cellular genome in HBV-transfected HepG2 cells were also detected, which could indicate HBV replication[4]. In view of the intimate relationship between HBV infection and hepatoma, relationship between HBV and p53 is always a research spot. In this experiment, we observed the effect of HBV on p53 expression and activity in SMMU-7721 cell line.
Plasmids and cell Human hepatoma cell line, SMMU-7721, was constructed in our laboratory, which can express wild type p53, but without HBV replication. The plasmids of pCMVHBVa (wild type HBV), pCMVHBVb (mutant type HBV, C-T substitution at 1 653 bp) and pCMVp53 (containing wild type p53 1.8 kb cDNA) were supplied by the Center for Human Virology of Thomas Jefferson University. PG13-CAT was kindly provided by Dr. Vogelstein, which contains 13 consecutive p53 recognition sites, making the expression of chloramphenicol acetyltransferase (CAT) regulated by p53 protein in the cells, and its inner control plasmid was pSVb-gal (Promega, USA). p21-luc (kindly provided by Dr. Vogelstein) contains p53 binding site with p21 promoter and luciferase reporter gene. The inner control plasmid PRL-SV40 (Promega) was cotransfected into cells to eliminate the difference caused by transfection efficiency or cell counts.
Chemicals and reagents LipofectamineTM transfection reagent was purchased from Gibco BRL (Gaithersburg, MD, USA). Dual Luciferase Assay System was purchased from Promega (USA). Annexin-V-FITC reagent was from Bender MedSystems (Vienna, Austria), CAT-ELISA kit was purchased from Roche (Mannheim, Germany). BCA protein detection kit was from Tian-Cheng Company (Shanghai, China). Protein prestained marker was provided by BioLabs (Australasia). Western blotting luminal reagent was from Santa Cruz (USA). Mouse mAb P53-DO7 was from Antibody Diagnostica Incorporation (USA). Internal control b-actin antibody was from Sigma (USA). Goat-anti-mouse horseradish peroxidase antibody and PVDF were from Hua-Mei Biological Company (Shanghai, China).
Cell culture and transient transfection with plasmid DNA SMMU-7721 cells were cultured in DMEM media supplemented with 100 mL/L fetal calf serum (FCS) and antibiotics, and incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2. The experiment was divided into four groups: pCMVp53 was transfected alone or cotransfected with pCMVHBVa or pCMVHBVb into the SMMU-7721 cell; and the control group was transfected by sperm DNA. The total of 2 μg DNA was transfected into the cells in each group. DNA amount was held constant in all experiments by adjusting with sperm DNA. Plasmid DNAs were introduced into SMMU-7721 by lipofectamineTM following the manufacturer's instructions. Briefly, cells were seeded into 35-mm culture dishes for 24 h and the medium was refreshed 4 h before transfection. The DNA mixed with 12 μL of lipofectamineTM was added to each plate and incubated in serum-free medium at 37 °C for 6.5 h, and then the medium was replaced with RPMI containing 100 mL/L FCS. Sixty hours after transfection, cells were observed under microscope. Cells were harvested to detect the apoptotic rate by flow cytometry or p53 expression by Western blot.
CAT ELISA Cells were prepared and transfected as aforementioned. Total amount (2.0 μg) of DNAs were kept constant by adding PG13-CAT. SV40-driven b-gal internal control plasmid and the respective test plasmids were as follows: pCMVHBVa, pCMVHBVb, pCMVp53, or the control sperm DNA. Before harvesting, the cells were washed thrice with PBS and extracted following the manufacturer's instructions. b-Galactosidase activity was determined in 96-well plates. A total of 200 μL aliquot of the clear lysates was tested for CAT concentration in the CAT ELISA according to the manufacturer's instructions (Roche Molecular Biochemicals). The CAT concentration of each sample was normalized with respect to b-gal activity.
Luciferase assays Transfections were carried in triplicate. Exponentially growing cells were plated at a density of 5?04/well in 0.5 mL medium into 24-well plates. Cells were cultured overnight before transfection. Then 300 ng of luciferase reporter constructs were cotransfected with 300 ng of constructs expressing wild-type p53 proteins or pCMVHBVa or pCMVHBVb. Renilla luciferase expression vector (3 ng) was regarded as an internal control. DNA amount was held constant in all experiments by adjusting with sperm DNA. The cells were harvested 60 h after transfection by lysis with passive lysis buffer (Promega). Firefly and Renilla luciferase activities were assayed using Dual Luciferase Assay System (Promega) according to the manufacturer's instructions. The firefly luciferase activity was normalized to Renilla luciferase activity to compensate for variability in transfection efficiencies.
Flow cytometric analysis of apoptotic cells The cells were stained with FITC-labeled annexin V (Bender MedSystems) and propidium. FITC-labeled annexin V has been used to detect apoptotic cells because annexin binds PS exposed to the outer membrane in apoptotic cells[5-7]. Flow cytometric analysis was performed with a Becton Dickinson flow cytometer (FACScan), and the results were analyzed with the CELLQUEST program (Becton Dickinson).
Western blot analysis Cell pellets were lysed in lysis buffer containing protease inhibitors. The protein concentration of each cell lysate was determined with the BCA assay kit. Eighty micrograms of total protein was separated in a 100 g/L SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride transfer membrane according to standard procedures. Western blotting using anti-p53 mouse mAb DO-7 (1:1 000 dilution) was performed. ECL internal control protein b-actin detection was performed simultaneously.
The results were expressed as mean±SD. Differences between experimental groups were evaluated by Student's t-test. P value <0.05 was considered statistically significant.
Expression of p53 has an influence on the p21 promoter. As a control, we first tested whether wild-type p53 protein could activate transcription from a p21 promoter. Wild-type p53 triggered an increase in transcription from the p21 promoter and the luciferase values increased significantly when pCMVHBVa was cotransfected, but not in the pCMVHBVb-cotransfected group (Table 1).
To determine the effect of HBV on the transcriptional activities of p53, p53 plasmid was transfected transiently alone or cotransfected with HBVa or HBVb, along with the PG13-CAT reporter construct (the CAT reporter gene under the control of a p53-responsive element). Even in the group transfected with PG13-CAT plasmid alone, a weak expression of CAT enzyme (1.272?.253) was observed, which suggested that there was an underlying transcription effect of p53 in SMMU-7721. However, in the group with pCMVp53 transfection-induced overexpression of p53, CAT activity was found to be greatly increased (3.585?.869). Moreover, the group cotransfecting HBVa with PG13-CAT also had a significantly increased CAT activity than that of the group transfected with PG13-CAT alone (2.559?.231), while cotransfection of HBVb had no marked influence on CAT activity of PG13-CAT (Table 2).
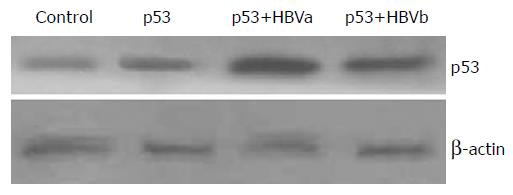
It was found that an increase in p53 transcription activity was associated with HBV replication, then it was interesting to examine whether it was caused by the upregulation of p53 protein expression. Our results showed that p53 had a weak expression in the normal SMMU-7721 cells. However, p53 expression was markedly increased after transfection with the pCMVp53 plasmid. Furthermore, cotransfection of pCMVp53 with pCMVHBVa showed an even higher p53 expression, while the change was not remarkable when pCMVHBVb was cotransfected with p53 (Figure 1).
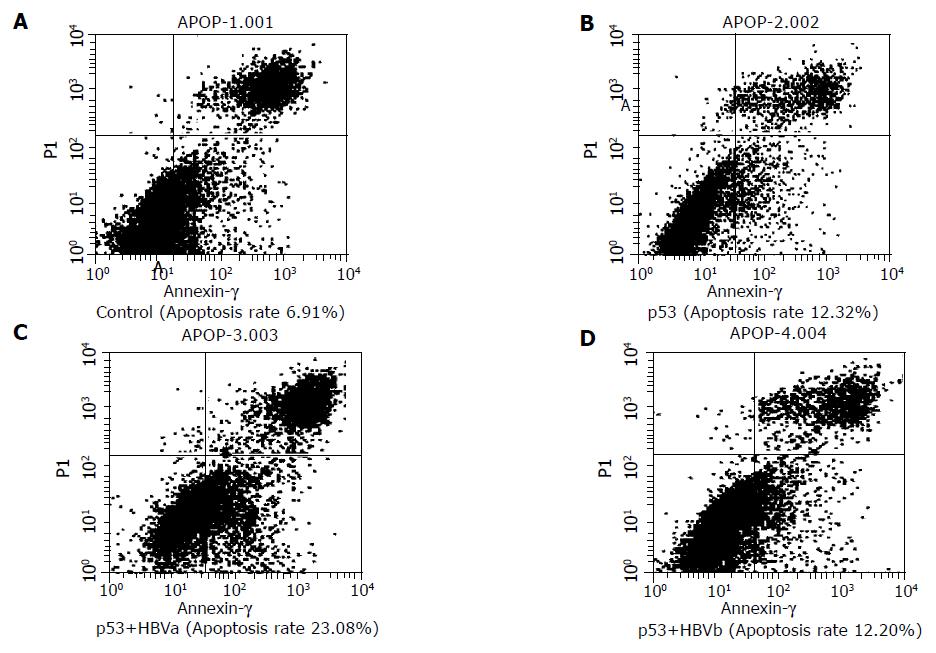
One of the important effects of p53 was its apoptosis-promoting effect. Thus, we observed the apoptosis rate which, in some sense, could indicate p53 activity. We observed that pCMVp53-transfected group had a significantly higher apoptosis rate than the control group (12.32% vs 6.91%). There was a much higher apoptosis rate in the pCMVp53 and pCMVHBVa cotransfected group (23.08%), but not in the pCMVp53 and pCMVHBVb cotransfected group (12.20%, Figure 2).
Although several data have suggested that the transformation process and tumorigenesis result from activation of oncogenes and inactivation of tumor suppressor genes of the DNA repair system, the question remains of what applies to PHC development. Despite the long-standing association between HBV and PHC development, the role of HBV in the transformation process is still unknown.
The long list of loci with frequent mutations associated with PHC suggests that the mechanism generating these genetic changes might be important in PHC development and needs to be identified. Mutation or inactivation of tumor suppressor gene p53 and its downstream gene p21WAF1 may have an implication in the PHC development[8]. Under normal conditions, p53 is most probably latent. Exposure of cells to p53-activating signals can lead, within a relatively short time, to a marked elevation of p53 protein.
In this experiment, HBV and p53 were transiently transfected into SMMU-7721 cell in order to investigate the apoptosis of the cells, p53 expression, and activity of p53 downstream gene-p21WAF1 promoter and the replication of HBV. Our results showed that the transfection of p53 alone in SMMU-7721 cells could increase p21-promoter activity and the apoptosis rate of the cells, suggesting that p53 could promote p21 transcription and contribute to the apoptosis process of the cells. While cotransfection of wild-type HBV markedly increased both p53 protein expression and its transcription activity, and simultaneously, the cell apoptosis rate was significantly increased. In contrast, cotransfection of mutant HBV did not show any effect.
As we know, wild-type p53 is usually a very labile protein, turning over with a half-life sometimes as short as few minutes[9]. On contrary, mutant p53 has a longer half-life, which makes its accumulation in cells. It is generally accepted that the accumulation of active p53 in response to stress occurs mainly through post-translational mechanism, which stabilizes the p53 and increases the ability of nucleolus location[10]. The current results showed that HBV may contribute to promote the stability and activity of p53.
Shi et al[11] showed that p21 (WAF1/CIP1) expression was inversely correlated with p53 expression in hepatitis C virus (HCV)-related hepatocellular carcinomas (HCCs), but not with HBV-related HCCs and HCCs without viral infection, suggesting that different modes of p21 (WAF1/CIP1) regulation are involved in HCCs, differing from their hepatitis viral infection status, and p21 (WAF1/CIP1) expression appears to be predominantly related to altered p53 in HCV-related HCCs. Livezey et al[12] analyzed the p21/Waf1 protein expression in several different kinds of cells by Western blot and indicated that p21/Waf1 protein was upregulated in cells that replicate HBV, but there was still no reasonable interpretation about this phenomenon. Since p21WAF1/CIP1 (p21) is a universal inhibitor of cyclin-dependent kinases and is regulated transcriptionally by p53, which is activated by DNA stress, its expression reflects DNA stress in chronic hepatitis. We observed the correlationship of HBV with p53 and p21, raising the possibility that HBV may have an effect on p21 expression through p53 upregulation, thereby leading to the cell apoptosis.
It has been reported that expression of p21WAF1 in the hepatic disease may be correlated with the disease progression. p21 expression has been reported to be upregulated by the stress of inflammation and fibrosis, and might be influenced by viral proteins in human chronic liver disease. Once the liver develops HCC, the p21 mRNA expression decreases to prominently low levels[13]. In hepatocarcinogenesis induced by HBV and aflatoxin B in tree shrews, the immunopositivity for p21 was found before HCC development, which suggested that p21 protein might be an early marker in the development of HCC[14]. The upregulated p21 expression may play a role as a guard to prevent hepatocytes from tumorgenicity in hepatitis.
Most of the previous reports deemed that apoptosis could not happen to the carcinogenesis cells and/or such cells had no response to the death signal, which lead to unlimited cell growth. But recent research indicates that tumor cells not only exhibited excessive proliferation, but also underwent apoptosis at rates that far exceed those in normal tissue. Proliferation and apoptosis of the tumor cells coexist at all stages and the rates of cell replication are higher than that of apoptosis, allowing a preferential net gain of pre-neoplastic cells[15]. Though HBV chronic infection is an important risk factor of HCC development, HBV-related hepatocarcinoma often happens after dozens of years of infection. In this study, we found that transient transfection of HBV induced apoptosis of SMMU-7721 cells and inactively proliferation. Thus, it is considered that there may be a balance between proliferation and apoptosis in HBV-infected hepatic cells. Hence, disruption of this balance, that is, predominance of proliferation over apoptosis, leads to tumorigenesis.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
Co-first-authors: Jian-Hui Qu and Ming-Hua Zhu
| 1. | Harris CC. p53: at the crossroads of molecular carcinogenesis and risk assessment. Science. 1993;262:1980-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 304] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Zhu MH. Department of Pathology, Fourth Military Medical University, Xi’an. Zhonghua Yixue Zazhi. 1993;73:325-328. |
| 3. | Feitelson MA, Zhu M, Duan LX, London WT. Hepatitis B x antigen and p53 are associated in vitro and in liver tissues from patients with primary hepatocellular carcinoma. Oncogene. 1993;8:1109-1117. [PubMed] |
| 4. | Livezey KW, Negorev D, Simon D. Hepatitis B virus-transfected Hep G2 cells demonstrate genetic alterations and de novo viral integration in cells replicating HBV. Mutat Res. 2000;452:163-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;82:1545-1556. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2108] [Cited by in RCA: 2148] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 6. | Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415-1420. [PubMed] |
| 7. | Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3792] [Cited by in RCA: 4020] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 8. | Hussain SP, Hollstein MH, Harris CC. p53 tumor suppressor gene: at the crossroads of molecular carcinogenesis, molecular epidemiology, and human risk assessment. Ann N Y Acad Sci. 2000;919:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Rogel A, Popliker M, Webb CG, Oren M. p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985;5:2851-2855. [PubMed] |
| 10. | Meek DW. Mechanisms of switching on p53: a role for covalent modification? Oncogene. 1999;18:7666-7675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Shi YZ, Hui AM, Takayama T, Li X, Cui X, Makuuchi M. Reduced p21(WAF1/CIP1) protein expression is predominantly related to altered p53 in hepatocellular carcinomas. Br J Cancer. 2000;83:50-55. [PubMed] |
| 12. | Livezey KW, Negorev D, Simon D. Hepatitis B virus-transfected Hep G2 cells demonstrate genetic alterations and de novo viral integration in cells replicating HBV. Mutat Res. 2000;452:163-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Wagayama H, Shiraki K, Yamanaka T, Sugimoto K, Ito T, Fujikawa K, Takase K, Nakano T. p21WAF1/CTP1 expression and hepatitis virus type. Dig Dis Sci. 2001;46:2074-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Su JJ, Ban KC, Li Y, Qin LL, Wang HY, Yang C, Ou C, Duan XX, Lee YL, Yang RQ. Alteration of p53 and p21 during hepatocarcinogenesis in tree shrews. World J Gastroenterol. 2004;10:3559-3563. [PubMed] |
| 15. | Grasl-Kraupp B, Ruttkay-Nedecky B, Müllauer L, Taper H, Huber W, Bursch W, Schulte-Hermann R. Inherent increase of apoptosis in liver tumors: implications for carcinogenesis and tumor regression. Hepatology. 1997;25:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |