Published online Oct 21, 2005. doi: 10.3748/wjg.v11.i39.6208
Revised: April 2, 2005
Accepted: April 5, 2005
Published online: October 21, 2005
AIM: To study the effects of microcirculation disturbance (MD) on rats with acute severe pancreatitis (ASP).
METHODS: We developed ASP rat models, and anatomized separately after 1, 3, 5, 7, and 9 h. We took out blood and did hemorrheologic examination and erythrocyte osmotic fragility test, checked up the water content, capillary permeability, and genetic expression of intercellular adhesion molecule-1 (ICAM-1) in lung tissues, examined the apoptosis degree of blood vessel endothelium while we tested related gene expression of Bax and Bcl-2 in lung tissues. We did the same examination in control group.
RESULTS: The viscosity of total blood and plasma, the hematocrit, and the erythrocyte osmotic fragility were all increased. Fibrinogen was decreased. The water content in lung tissues and capillary permeability were increased. Apoptosis degree of blood vessel endothelium was increased too. ICAM-1 genetic expression moved up after 1 h and reached its peak value after 9 h.
CONCLUSION: MD plays an important role in ASP following acute lung injury (ALI). The functional damage of blood vessel endothelium, the apoptosis of capillary vessel endothelium, WBC edging-concentration and the increasing of erythrocyte fragility are the main reasons of ALI.
- Citation: Liu XM, Liu QG, Xu J, Pan CE. Microcirculation disturbance affects rats with acute severe pancreatitis following lung injury. World J Gastroenterol 2005; 11(39): 6208-6211
- URL: https://www.wjgnet.com/1007-9327/full/v11/i39/6208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i39.6208
Microcirculation change has a great effect on causing diseases and acute severe pancreatitis (ASP) process. The lung can be damaged easily[1]. About 70% of deaths are due to acute respiratory distress syndrome[2,3]. In this study, we observed the microcirculation in rats and the changes in lung. The relationship between acute lung injury (ALI) and microcirculation change in rats with ASP was studied.
Rats were provided by the Experimental Animal Center of Medical School of Xi'an Jiaotong University. Experiment group (40 rats) was injected with 4% sodium taurocholate 1 mL/kg by puncturing pancreatic duct to make ASP models. Control group (eight rats) was only turned over duodenum. Experiment group was divided into five subgroups, eight rats in each group. They were anatomized after 1, 3, 5, 7, and 9 h, respectively.
Blood sedimentation (BS) was done with 0.2 mL venous blood. After centrifugation, hematocrit was read. The content of fibrinogens (CB) was recorded after water bath and centrifugation. The viscosity of total blood (VTB) and plasma (VP) was checked up in another 1-2 mL venous blood. The resistance of RBC to different concentrations of hypo-osmotic saline was examined. The permeability fragility of RBC was detected.
Lung tissues were fixed and sliced. Apoptosis staining experiment was done. We observed the change of lung capillary vessel endothelium apoptosis under a light microscope. The cells with brown-blue nuclei were considered as apoptosis cells.
The cells stained brown or brown-yellow were positively stained cells. To account the positive staining, we counted four high-power fields according to the formula: positive rate = the number of positive cells/total number of cells?00%. It was determined as negative staining (-) when the positive rate was <15%, and as positive staining when the positive rate was 15-50% (+), 50-75% (++), and >75% (+++), respectively.
The change of lung capillary permeability was detected by injecting Evan's blue into veins. Using a 625-nm spectropho-tometer, the concentration of Evan's blue represented the milligrams of the dyes per gram of wet weight lung.
First, we weighed the wet weight of lung tissues, and after being parched for 72 h at 70 °C, we weighed the dry weight. The water content (%) = (wet weight-dry weight)/wet weight.
We took b-actin as an inner contrast gene. Lung RNA was isolated by TRIzol. Oligo(dT)15 was used as a primer. The reaction conditions were at 42 °C for 15 min, at 99 °C for 5 min, at 4 °C for 4 min, and then we got the first chain of cDNA. PCR was done for 1 min at 94 °C, for 1 min at 55 °C, for 2 min at 72 °C, 35 circles in all. Finally, it was expanded for 10 min at 72 °C. After electrophoresis, we confirmed the quantity with a white/ultraviolet transilluminator. The expanding quantity of targeted genes/the expanding quantity of inner contrast genes expressed the quantity of detected genes.
The data were expressed as mean±SD. Kruskal-Wallis method and Mann-Whitney method were adopted to compare the two indexes.
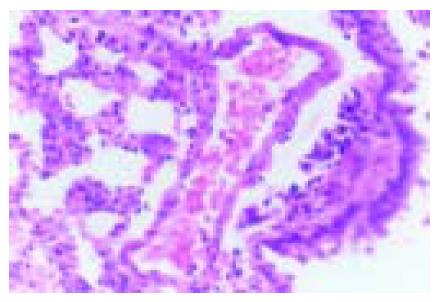
We observed the pathological change of lung tissues and found that interstitial pulmonary edema, focal and congestive pulmonary atelectasis were present at the forepart of ASP. PMNs were present in capillaries, and thrombosis was visible in capillaries (Figure 1). The changes aggravated gradually.
Apoptosis endothelium could be examined in lungs of control group. After the models were made, the number of apoptosis endothelial cells was increased. When we compared the number of apoptosis cells at 3 h with that at 9 h, we found that the number of apoptosis endothelial cells at 9 h was greater than that at 3 h. This trend was related to the grade of ALI.
Bcl-2 and Bax protein were less expressed in normal lung tissues. After ASP models were made, we found that Bcl-2 and Bax protein expression was obvious. Bax protein expression accorded with the changes of cell apoptosis, and reached its peak in 7-9 h. Bcl-2 expression was inversely correlated with the changes of cell apoptosis, and declined gradually after 3 h (Table 1).
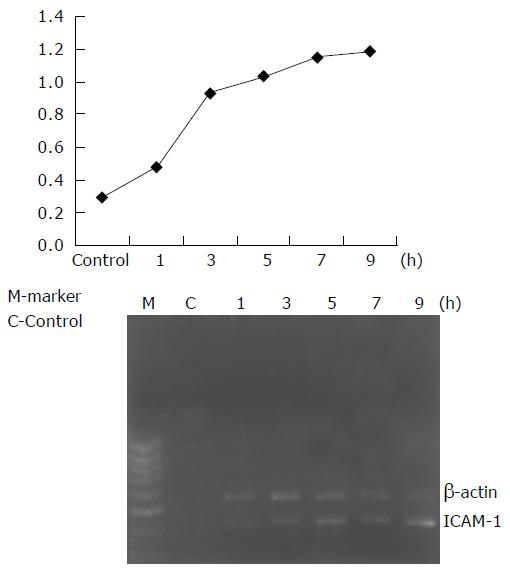
The genetic expression of intercellular adhesion molecule-1 (ICAM-1) in lung tissues was increased after 1 h and reac-hed its peak after 9 h. The gene expressions of ICAM-1 were significantly different in each period of time (P<0.05, Figure 2).
Compared with control group, the water content and capillary permeability in lung tissues in ASP group were both increased obviously in each period of time (Table 2).
Compared with control group, each items of hemorrheology were diverse in each period of time (Table 3).
| control | 1h | 3h | 5h | 7h | 9h | |
| High VTB (mPas)b | 5.65±1.04 | 7.66±0.90 | 7.81±0.41 | 7.79±0.32 | 8.32±0.46 | 9.49±1.34 |
| Low VTB (mPas)b | 14.57±4.00 | 22.72±2.26 | 23.77±3.13 | 22.80±1.61 | 29.46±4.81 | 30.91±7.28 |
| VP(mPas)b | 3.57±0.49 | 5.10±0.87 | 4.56±1.08 | 5.76±0.88 | 6.25±1.00 | 8.00±1.34 |
| BS(mm.h-1)b | 9.33±0.82 | 0.90±0.11 | 0.59±0.31 | 0.33±0.15 | 0.12±0.11 | 0.03±0.03 |
| haematocrit (%)b | 44.92±2.73 | 56.00±2.59 | 57.17±2.88 | 57.67±1.86 | 59.17±2.96 | 62.17±3.39 |
| CB(g / L)b | 452.3±29.9 | 145.0±45.4 | 142.0±14.6 | 88.3±20.8 | 67.4±17.7 | 51.3±19.1 |
| original hemolysisb | 0.45±0.02 | 0.47±0.02 | 0.47±0.02 | 0.47±0.02 | 0.47±0.02 | 0.49±0.02 |
| complete hemolysisb | 0.36±0.00 | 0.38±0.02 | 0.39±0.02 | 0.40±0.03 | 0.41±0.02 | 0.43±0.02 |
The main characteristic of acute pancreatitis is the change in blood circulation of pancreas and the whole body[4-6]. Blood volume declined obviously in early period of ASP. Hemoperfusion could exacerbate ASP. In this experiment, sodium taurocholate was injected through the pancreatic duct to make ASP models. We observed various indexes of ASP at different time points. Significant hemorrheology changes could be observed in rats with ASP. The blood viscosity and the erythrocyte osmotic fragility were increased. The capillary permeability in lung tissues was also increased. Interstitial pulmonary and microthrombosis were found. Obvious microcirculation disturbance (MD) could be observed in lung tissues and the whole body of rats with ASP. After the models were made, the number of apoptosis endothelial cells and the genetic expression of ICAM-1 in lung tissues were significantly increased, suggesting that ASP can cause MD in many ways and plays an important role in causing and exacerbating ALI.
WBCs adhered to the blood vessel endothelium could cause inflammation[7-9]. Adhesion molecule could play an important role in adhesion process, and its expression could reflect the change of blood vessel endothelium[10-17]. After ASP models were made, ICAM-1 gene expression was increased and became easier for WBCs to adhere to blood vessel endothelium. Therefore, it made WBCs to invade the lung tissues more easily.
For vein wall permeability, blood vessel endothelium is the main barrier. In this study, the number of apoptosis cells increased and apoptosis was accorded with the increasing of lung tissues, vessel permeability and water content. We think that the increasing of apoptosis cells in lung tissues could alter the structure integrality of capillary endothelium. Because the endothelial cells could not renew rapidly by themselves, apoptosis could lead to decrease of blood vessel endothelial cell storage. This kind of change results in the increase of blood vessel endothelium gap and its permeability[18,19].
Our study found that hemorrheology changed obviously in blood of ASP rats, thus leading to more serious MD of pancreas and other organs. This is the main reason for pancreas necrosis and ARDS representation[20,21]. The primary presentations are the decline of RBC aggregation capacity, increase of VTB and VP as well as WBC edging-concentration. The later is the primal step of WBCs swimming outside of blood vessel, leading to grievous inflammation and ALI[22]. WBC edging-concentration in rats with ASP was affected not only by inflammation medium and chemotatic factor but also by other factors, such as tiny vein, blood viscosity, and RBC aggregation[23].
The erythrocyte fragility was increased after ASP presentation, and became more serious following the progress of the disease. The increase of erythrocyte fragility could reflect the defective capacity of RBC distortion. RBCs break up easily, and release adenosine diphosphate, thus causing the activation of coagulation system and disseminated intravascular coagulation, leading to the decrease of fibrinogen.
In conclusion, MD plays an important role in the process of ASP with lung injury. Ameliorating MD could not only reduce local damages in pancreas, but also protect the lung and other organs in the body. Therefore, ameliorating MD is an effective therapy in treating ASP.
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
| 1. | Zhu AJ, Shi JS, Sun XJ. Organ failure associated with severe acute pancreatitis. World J Gastroenterol. 2003;9:2570-2573. [PubMed] |
| 2. | Liu XM, Pan CE, Liu QG, Wang ZF. The diagnosis and treatment of lung injury following severe acute pancreatitis. Zhonghua Gandan Waike Zazhi. 2002;8:116-117|. |
| 3. | Zhao M, Chen RF. The pathogenesis of lung injury following acute necrotizing pancreatitis. Shijie Huaren Xiaohua Zazhi. 2001;9:954-957. |
| 4. | Foitzik T, Eibl G, Hotz B, Hotz H, Kahrau S, Kasten C, Schneider P, Buhr HJ. Persistent multiple organ microcirculatory disorders in severe acute pancreatitis: experimental findings and clinical implications. Dig Dis Sci. 2002;47:130-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Zhou Z, Zhang Z, Yan L, Shu Y, Cheng Z, Zhao J, Lan P, Feng X, Wang R. [The feature of pancreatic microcirculatory impairment in caerulein induced acute pancreatitis]. Zhonghua Wai Ke Za Zhi. 1999;37:138-40, 9. [PubMed] |
| 6. | Xia SH, Zhao XY, Guo P, Da SP. Hemocirculatory disorder in dogs with severe acute pancreatitis and intervention of platelet activating factor antagonist. Shijie Huaren Xiaohua Zazhi. 2001;9:550-554. |
| 7. | Chen H, Li F, Cheng YF, Sun JB. Pathogenic role of neutrophils in evolution of acute pancreatitis in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:776-779. |
| 8. | Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Kyriakides C, Jasleen J, Wang Y, Moore FD, Ashley SW, Hechtman HB. Neutrophils, not complement, mediate the mortality of experimental hemorrhagic pancreatitis. Pancreas. 2001;22:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Sun W, Zhang JD, Zhao Y, Zhao Y, Wang Q. Expression of IL-6 and integrin family cell adhesion molecules in acute necrotizing pancreatitis complicated with multiple organ injury in rats. Shijie Huaren Xiaohua Zazhi. 2003;11:753-755. |
| 11. | Shi L, Tian FZ, Huang DR, Li X, Zhao B, Gu DY, Tang XD, Wang Y. Effect of hepatic NF-kB on ICAM-1 expression in rats with acute pancreatitis. Shijie Huaren Xiaohua Zazhi. 2003;11:1505-1507. |
| 12. | Yi Y, Gao NR. The celluliar adhering elements and acute pancreatitis. Shijie Huaren Xiaohua Zazhi. 2001;9:70-71. |
| 13. | Lundberg AH, Fukatsu K, Gaber L, Callicutt S, Kotb M, Wilcox H, Kudsk K, Gaber AO. Blocking pulmonary ICAM-1 expression ameliorates lung injury in established diet-induced pancreatitis. Ann Surg. 2001;233:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Nakae H, Endo S, Sato N, Wakabayashi G, Inada K, Sato S. Involvement of soluble adhesion molecules in acute pancreatitis. Eur Surg Res. 2001;33:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Powell JJ, Siriwardena AK, Fearon KC, Ross JA. Endothelial-derived selectins in the development of organ dysfunction in acute pancreatitis. Crit Care Med. 2001;29:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Telek G, Ducroc R, Scoazec JY, Pasquier C, Feldmann G, Rozé C. Differential upregulation of cellular adhesion molecules at the sites of oxidative stress in experimental acute pancreatitis. J Surg Res. 2001;96:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, Steer ML. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Liu XM, Liu QG, Pan CE, Xu GF, Zhang T, Huang JY, Zhang M. Influence of apoptosis of endothelial cell on lung injury following severe acute pancreatitis in rats. Disi Junyi Daxue Xuebao. 2003;24:1677-1679. |
| 19. | Wu R, Song X, Xu Y, Meng X. [Apoptosis of endothelial cells in alteration of microvascular permeability in lung during sepsis]. Zhonghua Wai Ke Za Zhi. 2000;38:385-387. [PubMed] |
| 20. | Wang ZF, Pan CE, Liu SG, Liang GG, Zhang M. The significance of hemorrheologic alternation in acute necrotizing pancreatitis. Zhongguo Putong Waike Zazhi. 2000;9:225-227. |
| 21. | Wang ZF, Pan CE, Liang GG, Liu SG, Zhang M. The treatment of acute necrotizing pancreatitis by modifying hemorrheologic alternation. Zhonghua Gandan Waike Zazhi. 2000;6:89. |
| 22. | Zhou YK, Wu Y. The correlation between blood rheology and multiple organic injury in acute pancreatitis. Shijie Huaren Xiaohua Zazhi. 2000;8:1055-1057. |
| 23. | Liang GG. The study of the relationship between apparent vislosity and the aggregation of erythrocytes when the blood performs a tubular steady laminal flow with small pressure difference. Xi'an Yike Daxue Xuebao. 2000;21:259-262. |