INTRODUCTION
In 1998, star-shaped cells in the pancreas, namely pancreatic stellate cells (PSCs), were identified and characterized[1,2]. In normal pancreas, stellate cells are quiescent and can be identified by the presence of vitamin A-containing lipid droplets in the cytoplasm. In response to pancreatic injury or inflammation, they are transformed ("activated" from their quiescent phenotype into myofibroblast-like cells, which actively proliferate, express the cytoskeletal protein a-smooth muscle actin, and produce extracellular matrix components. Many of the morphological and metabolic changes associated with the activation of PSCs in animal models of fibrosis also occur when these cells are grown in serum-containing medium in culture on plastic. There is accumulating evidence that activated PSCs play a pivotal role in the development of pancreatic fibrosis[1-4]. In addition, PSCs may participate in the pathogenesis of acute pancreatitis[3,5]. The activation of signal transduction pathways such as p38 mitogen-activated protein (MAP) kinase[6], Rho-Rho kinase[7], and c-Jun N-terminal kinase[8] is likely to play a role in PSC activation. But intracellular signal transduction pathways in PSCs remain largely unknown.
Endothelin-1 (ET-1), which acts through G-protein coupled ETA and ETB receptors, was initially described as a potent endothelial cell-derived vasoconstrictor isolated from the culture medium of porcine aortic endothelial cells[9]. Subsequent studies have shown that ET-1 is produced by a variety of cells and exhibits several biological activities such as vasoconstriction, regulation of peptide secretion, migration, and positive or negative effects on cell growth[10,11]. ET-1 has been implicated in the pathogenesis of cardiovascular, pulmonary, renal, and developmental disorders[11]. In addition, ET-1 plays a key role in the development of portal hypertension and hepatic fibrosis through its effects on hepatic stellate cells[12,13]. ET-1 has been shown to stimulate contraction via ETA receptor[14], and inhibit proliferation via ETB receptor[15] in hepatic stellate cells. But, the roles of ET-1 and ET receptors in the regulation of cell functions of PSCs remain largely unknown. In this study, we examined the ability of ET-1 to affect the cell functions of PSCs and the underlying molecular mechanisms. We here report that ET-1 stimulated contraction and migration, but not proliferation, of PSCs through ET receptors and Rho-Rho kinase pathway.
MATERIALS AND METHODS
Materials
ET-1 was purchased from Peptide Institute (Osaka, Japan). Collagenase P was obtained from Roche Diagnostics (Mannheim, Germany). Rat recombinant platelet-derived growth factor (PDGF)-BB was purchased from R&D Systems (Minneapolis, MN). Rhodamine-labeled phalloidin was purchased from Molecular Probes (Eugene, OR). BQ-123, BQ-788, and U0126 were purchased from Calbiochem (La Jolla, CA). Y-27632 was a generous gift from Mitsubishi Pharma Co. (Osaka, Japan). Rabbit antibodies against extracellular-signal regulated kinase (ERK; phosphorylated and total), Akt (phosphorylated at Ser473 and total), and myosin regulatory light chain II (MLC; phosphorylated at Ser19 and total) were purchased from Cell Technologies, Inc. (Beverly, MA). Rabbit antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Trevigen (Gaithersburg, MD). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specifically described.
Cell culture
All animal procedures were performed in accordance with the National Institutes of Health Animal Care and Use Guidelines. Rat PSCs were prepared from the pancreas tissues of male Wistar rats (Japan SLC Inc., Hamamatsu, Japan) weighing 200-250 g using the Nycodenz solution (Nycomed Pharma, Oslo, Norway) after perfusion with 0.3 g/L collagenase P as previously described[16]. The cells were resuspended in Ham's F-12 medium containing 100 mL/L heat-inactivated fetal bovine serum (MP Biomedicals, Irvine, CA), penicillin sodium, and streptomycin sulfate. Cell purity was always more than 90% as assessed by a typical star-like configuration and by detecting vitamin A autofluorescence. All experiments were performed using cells between passages two and five. We incubated PSCs in serum-free medium for 24 h before the addition of experimental reagents. For some experiments, BQ-123, BQ-788, U0126, or Y-27632 were added 30 min prior to the ET-1 treatment.
Reverse transcription-PCR
Total RNA was isolated using RNeasy total RNA preparation kit (Qiagen, Chatsworth, CA). Total RNA was reverse-transcribed, and the resultant cDNA was subjected to PCR as previously reported[17]. Specific primer sets were as follows (listed 5×3× forward and reverse, respectively). ET-1 precursor (preproET-1): TTGCTCCTGCTCCTC-CTTGAG and GGTCTTGATGCTGTTGCTGATG; ETA: CGTCTTCTGCTTGGTTGTCA and TGGTTCT-GCTCCTGGTTCTT; ETB CAAGACAGTATTCTGC CTGG and CAAGCAGGATTGCTTCTCCT. They were amplified using 30 cycles at 94 °C (for 1 min), at 50 °C (for 1 min), and at 72 °C (for 1 min). The products of PCR were separated by 15 g/L agarose gel electrophoresis and visualized under ultraviolet light after staining with ethidium bromide. The expected sizes of the PCR products were 382 bp for preproET-1, 321 bp for ETA receptor, and 297 bp for ETB receptor.
Immunostaining
PSCs were grown directly on slides, serum-starved for 24 h, and immunostaining for ET-1 was performed using a streptavidin-biotin peroxidase complex detection kit (Histofine Kit; Nichirei, Tokyo, Japan) as previously described[18]. Briefly, cells were fixed with 1 000 mL/L methanol at -20 °C, and then endogenous peroxidase activity was blocked by incubation with hydrogen peroxide in methanol. After immersion in normal rabbit serum, the slides were incubated with mouse anti-ET-1 antibody (at 1:200 dilution) at 4 °C overnight. The slides were incubated with biotinylated anti-mouse immunoglobulin antibody, followed by peroxidase-conjugated streptavidin. Finally, color was developed by incubating the slides for several minutes with diaminobenzidine (Dojindo, Kumamoto, Japan). The expression of ETA and ETB was examined in a similar manner.
Western blotting
The level of activated, phosphorylated ERK was determined by Western blotting as previously described[19]. Cells were lysed in sodium dodecyl sulfate buffer. Cellular proteins (approximately 100 mg) were fractionated on a 100 g/L sodium dodecyl sulfate-polyacrylamide gel. They were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA), and the membrane was incubated overnight at 4 °C with rabbit anti-phosphospecific ERK antibody. After incubation with peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody for 1 h, proteins were visualized using an ECL kit (Amersham Biosciences, UK). The levels of total ERK, Akt (phosphorylated and total), and MLC (phosphorylated and total) were determined in a similar manner.
Fluorescence microscopy
Stress fibers were stained with rhodamine-labeled phalloidin as previously described[20]. After fixation with 40 g/L paraformaldehyde for 5 min, PSCs were permeablized with 1.5 mL/L Triton X-100 in PBS for 5 min. Cells were then incubated for 2 h at room temperature in a dark place with rhodamine-labeled phalloidin in blocking buffer (10 g/L bovine serum albumin in PBS). Cells were then washed thrice with PBS for 10 min each, and coverslipped with Fluoromount (Vector Laboratories, Burlingame, CA). Cells were observed with a Leica fluorescence microscope. Images were captured with a Leica QFISH system (Wetzlar, Germany).
Collagen gel contraction assay
Contraction of PSCs on collagen lattices was examined in six-well flat-bottom tissue culture plates (Becton Dickinson, Bedford, MA) as previously described[21]. Culture vessels were preincubated with PBS containing 10 g/L bovine serum albumin for 2 h at 37 °C, washed, and air-dried. The gel mixture consisted of eight parts Vitrogen-100 (Collagen Corp., Palo Alto, CA), one part 10×minimal essential medium, and one part 0.2 mol/L HEPES, pH 9.0, which resulted in a final collagen concentration of 2.4 g/L. It was prepared at 4 °C, added to the culture vessel, and incubated at 37 °C for 2 h to allow gelation. PSCs were trypsinized, resuspended in serum-free medium (at 1×09 cells/L), plated on top of the gels (1 mL/well), and allowed to attach for 2 h. The collagen lattices were detached from the plate by gentle circumferential dislodgement using a fine needle, and ET-1 (at 100 nmol/L) was added to elicit contraction. After 24-h incubation, we measured the diameter of the collagen lattices, and calculated the " of the original diameter (= 35 mm)" In addition, cell-free collagen lattices were incubated with 100 nmol/L ET-1 or serum-free medium alone.
Cell migration assay
Cell migration was assessed as previously described[16]. Serum-starved PSCs were trypsinized, and resuspended at the concentration of 3×108 cells/L in serum-free medium containing 10 g/L bovine serum albumin. For the assay, we used modified Boyden chambers with 8-mm pore filters (Iwaki Glass Co. Ltd., Funabashi, Japan) coated with rat-tail type I collagen. ET-1 was added to the lower chamber, and 250 mL of cell suspension was added to the upper chamber. The chambers were then incubated at 37 °C for 24 h. At the end of the incubation, the cell suspension in the upper chamber was aspirated, and the upper part of the filter was cleaned with cotton plugs. The cells migrated to the underside of the filter were stained with Difquick (Sysmex, Kobe, Japan), viewed, and counted at 200×magnification.
Cell proliferation assay
Serum-starved PSCs (approximately 80% density) were treated with ET-1 in the presence or absence of 100 mL/L fetal bovine serum or PDGF-BB (at 25 mg/L). Cell proliferation was assessed using a commercial kit (Cell proliferation ELISA, BrdU; Roche Diagnostics) according to the manufacturer's instruction. This is a colorimetric immunoassay based on the measurement of 5-bromo-2×deoxyuridine (BrdU) incorporation during DNA synthesis. After 24-h incubation with experimental reagents, cells were labeled with BrdU for 3 h at 37 °C. Cells were fixed, and incubated with peroxidase-conjugated anti-BrdU antibody. Then the peroxidase substrate 3,3×5,5×tetramethylbenzidine was added, and BrdU incorporation was quantitated by A370-492.
Statistical analysis
The results were expressed as mean±SD. Luminograms and autoradiograms are representative of at least three experiments. Differences between the groups were evaluated by ANOVA, followed by Fisher's test for post hoc analysis. A P value less than 0.05 was considered statistically significant.
RESULTS
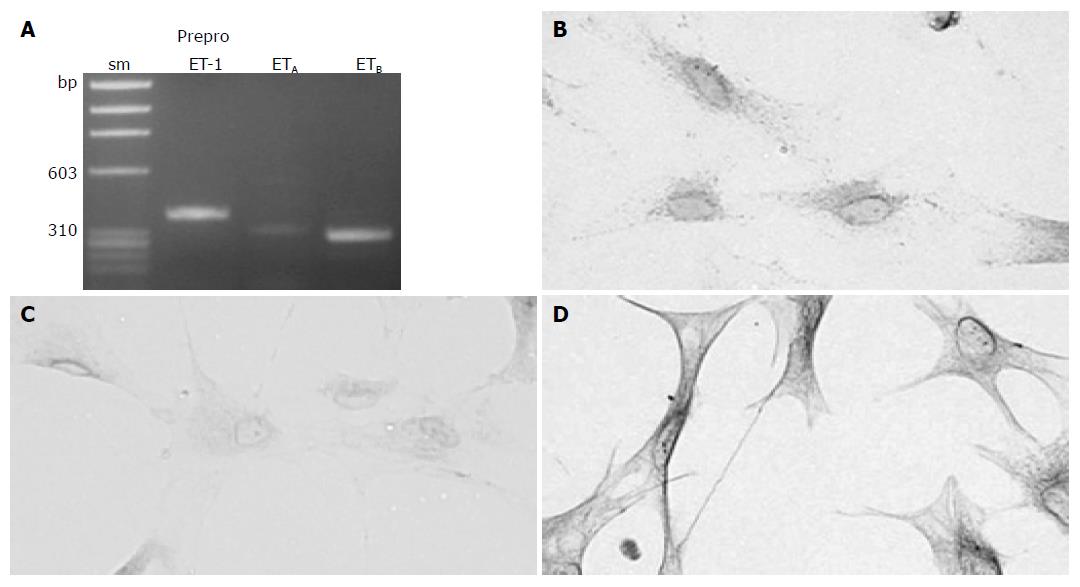
Activated PSCs expressed ET-1 and ET receptorsWe first examined whether culture-activated PSCs expressed ET-1 and ET receptors. ET-1 is a product of the gene coding for a large precursor protein (i.e. preproET-1)[9]. Culture-activated PSCs expressed ETA and ETB receptors, and ET-1, as assessed by reverse transcription-PCR and immunostaining (Figure 1).
Figure 1 Culture-activated PSCs expressed ET and ET receptors.
A: Total RNA was prepared from culture-activated, serum-starved PSCs. The expression of preproET-1, ETA receptor and ETB receptor was examined by reverse transcription-PCR. The expected sizes of the PCR products were 382 bp for preproET-1, 321 bp for ETA receptor, and 297 bp for ETB receptor. sm: size marker (fX174 HaeIII digest). bp: bp; B-D: Serum-starved PSCs were grown directly on slides. Immunostaining for ET-1 (panel B), ETA receptor (panel C), and ETB receptor (panel D) was performed using a streptavidin-biotin peroxidase complex detection kit. Original magnification: ×0 objective.
ET-1 induced phosphorylation of ERK and MLC, but not Akt
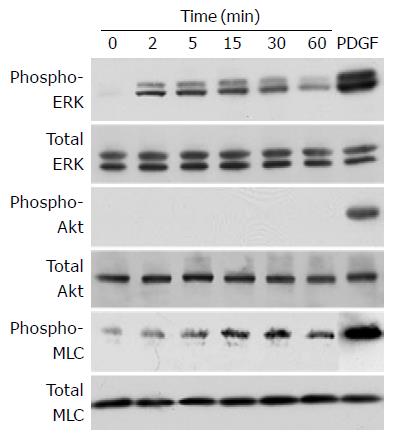
In an attempt to elucidate the effect of ET-1 on cell functions of PSCs, we examined whether ET-1 activated intracellular signaling pathways. We examined the activation of ERK, Akt, and MLC, all of which are implicated in ET-1-induced cellular contraction and/or migration[22-24]. PSCs were treated with ET-1 at 100 nmol/L, and the activation of the signaling pathways by Western blotting using anti-phosphospecific antibodies. We chose this ET-1 concentration because previous studies have shown that ET-1 acts on ET receptors in a dose-dependent manner and ET-1 at this concentration effectively elicited effects in a variety of cell types[14,15,25,26]. PDGF-BB induced phosphorylation of ERK, Akt, and MLC whereas ET-1 induced phosphorylation of ERK and MLC but not Akt (Figure 2).
Figure 2 ET-1 induced phosphorylation of ERK and MLC, but not Akt.
PSCs were treated with ET-1 (at 100 nmol/L) for the indicated time. Total cell lysates (approximately 100 mg) were prepared, and the levels of phosphorylated ERK, Akt, and MLC were determined by Western blotting. As positive controls, total cell lysates were prepared from PSCs treated with PDGF-BB (at 25 mg/L) for 5 min, and Western blotting was performed in a similar manner. Levels of total ERK, Akt, and MLC were also determined.
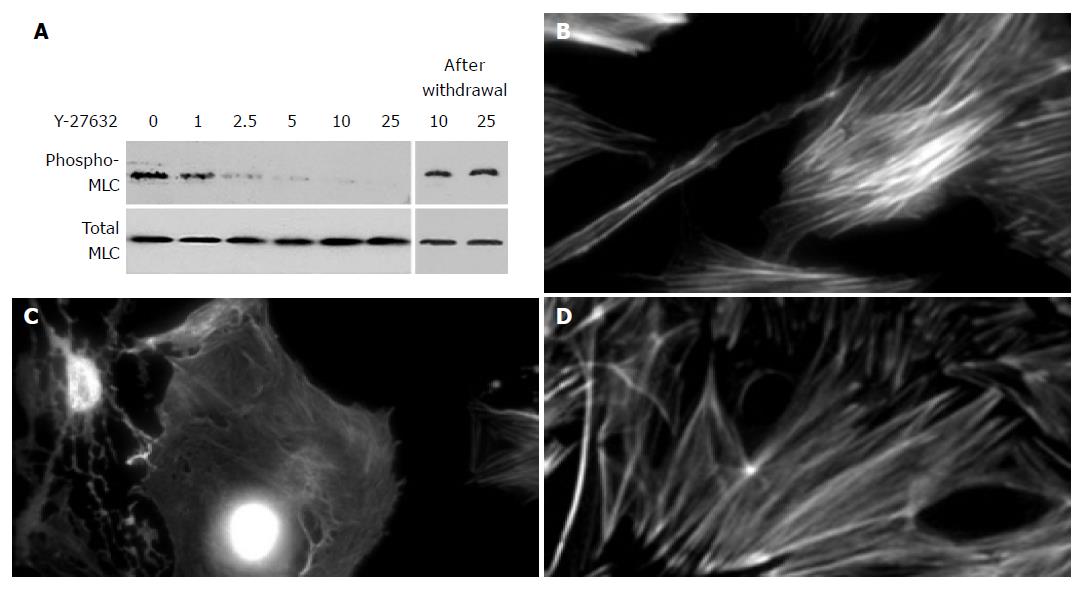
Y-27632 decreased stress fiber formation and MLC phosphorylation
It has been shown that RhoA, acting through its downstream effector Rho kinase, stimulates MLC phosphorylation, stress fiber formation, and contractile force generation[27,28]. The fact that ET-1 induced phosphorylation of MLC prompted us to examine the effect of Y-27632, a specific Rho kinase inhibitor[29], on the phosphorylation of MLC and stress fiber formation. Y-27632 decreased phosphorylation of MLC in a dose-dependent manner (Figure 3A). When cells were treated with Y-27632 at 25 mmol/L for 24 h, disassembly of stress fibers was observed (Figures 3B and 3C). In these experiments, Y-27632 up to 25 mmol/L did not affect the cell viability during the incubation as assessed by trypan blue exclusion test (data not shown). In addition, the effects of Y-27632 were reversible within 48 h after the removal of Y-27632 (Figures 3A and 3D). However, when PSCs were treated with Y-27632 at 50 mmol/L, cytotoxic effects were observed during the incubation (data not shown).
Figure 3 Y-27632 decreased stress fiber formation.
A: PSCs were treated with ET-1 (at 100 nmol/L) in the presence of Y-27632 at the indicated concentrations (at mmol/L) for 30 min. Total cell lysates (approximately 100 mg) were prepared, and the levels of phosphorylated and total MLC were determined by Western blotting. In parallel experiments, Y-27632 was withdrawn after 30-min incubation, and replaced with 10% FBS. After 48-h incubation with 10% FBS, total cell lysates were prepared, and the levels of phosphorylated and total MLC were determined by Western blotting; B-D: PSCs were incubated with ET-1 (at 100 nmol/L) in the absence (B) or presence (C) of Y-27632 (at 25 mmol/L) for 24 h, and stained with rhodamine-labeled phalloidin. In parallel experiments, Y-27632 was withdrawn and replaced with 10% FBS. After 48-h incubation with 10% FBS, PSCs were stained with rhodamine-labeled phalloidin (D). Original magnification: ×0 objective.
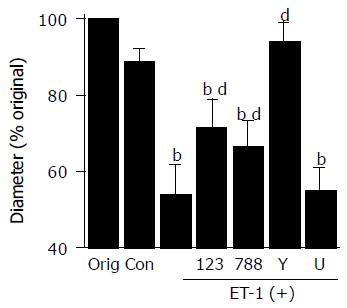
ET-1 induced contraction of PSCs
We examined whether ET-1 induced contraction of PSCs using collagen lattices. With no stellate cells, the size of the collagen lattices did not change in response to ET-1 (data not shown). When PSCs were plated on the lattices, and incubated with serum-free medium alone for 24 h, there was a slight reduction in the diameter of the lattices to 89×% of their original size (Figure 4A). By incubation with 100 nmol/L ET-1, the diameter of the lattices decreased to 54×% (Figure 4B). ET-1-induced contraction of PSCs was inhibited by an ETA receptor antagonist BQ-123[30], an ETB receptor antagonist BQ-788[31]. Y-27632 abolished ET-1-induced contraction, whereas a MAP kinase inhibitor U0126[32] was ineffective.
Figure 4 ET-1 induced contraction of PSCs.
PSCs were plated on the surface of collagen lattices prepared in each well of a six-well plate and incubated for 2 h to allow adherence. The collagen lattices were then detached from the plate by gentle circumferential dislodgment. Serum-starved PSCs were left untreated ("Con" or were treated with ET-1 (at 100 nmol/L) in the absence or presence of BQ-123 (×23×at 10 mmol/L), BQ-788 (×88×at 10 mmol/L), Y-27632 (at 25 mmol/L), or U0126 ("U"at 5 mmol/L). Lattices were then detached from the plate, and incubated in serum-free medium (control, "Con" with or without 100 nmol/L ET-1. After 24-h incubation, we measured the diameter of the collagen lattices, and calculated the × of the original diameter ("Orig"= 35 mm)× Data are shown as mean±SD (% of the original diameter, n = 8). bP<0.01 vs control (serum-free medium only), dP<0.01 vs ET-1 treatment only.
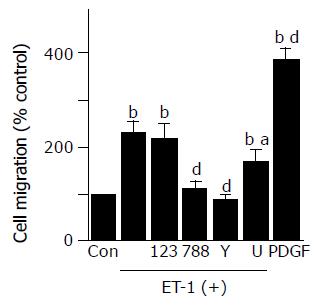
ET-1 induced migration, but did not alter proliferation of PSCs
ET-1 induced migration of PSCs, which was inhibited by BQ-788, Y-27632, and U0126, but not BQ-123 (Figure 5).
Figure 5 ET-1 induced migration of PSCs.
Cell migration was assessed using modified Boyden chambers with 8-mm pore filters. Serum-starved PSCs were left untreated ("Con" or were treated with ET-1 (at 100 nmol/L) in the lower chamber in the absence or presence of BQ-123 (×23×at 10 mmol/L), BQ-788 (×88×at 10 mmol/L), Y-27632 (at 25 mmol/L), or U0126 ("U"at 5 mmol/L). After 24-h incubation, the cells migrated to the underside of the filter were stained, and counted under a light microscopy. As positive controls, cells were treated with PDGF-BB (at 25 mg/L) in the lower chamber, and cell migration was assessed in a similar manner. Data are shown as mean±SD (% of the control, n = 6). bP<0.01 vs control (serum-free medium only), aP<0.05, dP<0.01 vs ET-1 treatment only.
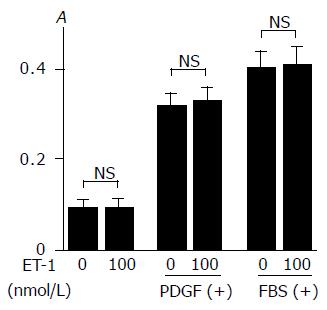
Previous studies have shown that ET-1 showed positive or negative effects on cell growth[15,25,26]. In agreement with the previous study[16], PDGF-BB and 100 mL/L fetal bovine serum increased proliferation of PSCs (Figure 6). ET-1 did not affect basal proliferation or proliferation in response to PDGF or serum.
Figure 6 ET-1 did not alter basal or inducible proliferation of PSCs.
Serum-starved PSCs were treated with ET-1 at 0 or 100 nmol/L in the absence or presence of PDGF-BB (at 25 mg/L) or 100 mL/L fetal bovine serum ("FBS". After 24-h incubation, DNA synthesis was assessed by BrdU incorporation enzyme-linked immunosorbent assay. Data are shown as mean±SD (n = 6). NS: not significant, A: optical density.
DISCUSSION
There is accumulating evidence that activated PSCs play a pivotal role in the pathogenesis of pancreatic fibrosis and inflammation[1-5]. We here showed that culture-activated PSCs expressed ETA and ETB receptors, and ET-1. ET-1 stimulated contraction and migration of PSCs, suggesting autocrine and paracrine mechanisms of ET-1-regulated cellular functions of PSCs. ET-1-induced contraction was inhibited by ETA and ETB receptor antagonists whereas ET-1-induced migration was inhibited by ETB receptor antagonist but not by ETA receptor antagonist. Thus, ETA and ETB receptors play different roles in the regulation of these cellular functions in response to ET-1.
Cellular migration and contraction are complex processes involving dynamic changes in the actin-myosin cytoskeleton, including generation of contractile forces[33]. It has been shown that contractile force is powered by myosin, which is activated by phosphorylation of MLC[33]. Phosphorylation of MLC is regulated by the balance of two enzymatic activities, i.e., MLC kinase(s) and myosin phosphatase. For example, MLC is phosphorylated by MLC kinase in a Ca2+/calmodulin-dependent manner in smooth muscle cells[34]. RhoA, acting through its downstream effector Rho kinase, stimulates MLC phosphorylation and consequently contractile force generation through the inhibition of myosin phosphatase[27,28]. Activated Rho kinase promotes the contraction of isolated stress fibers and generates a long-lasting tensile activity by the maintained inhibition of myosin phosphatase[35]. On the other hand, contractile force generation by certain non-muscle cell types including fibroblasts may occur independently of myosin phosphorylation[36,37]. To clarify the role of myosin in ET-1-induced contraction and migration of PSCs, we examined the effect of ET-1 on the phosphorylation of MLC. We here showed that ET-1 induced the phosphorylation of MLC, supporting a role of myosin in contractile force generation in PSCs. In addition, an inhibitor of Rho kinase Y-27632 abolished the phosphorylation of MLC, stress fiber formation, contraction, and migration. These results agree with the idea that force generation by the myofibroblasts depends on the isometric contraction of stress fibers containing a-smooth muscle actin, and is mediated through Rho-Rho kinase[38]. Indeed, myosin mediates contractile force generation by hepatic stellate cells in response to ET-1[24]. We have previously shown that PDGF-BB induced migration of PSCs through the activation of phosphatidylinositol 3-kinase/Akt pathway[16]. Unlike PDGF-BB, ET-1 did not activate Akt but did induce migration of PSCs, excluding a role of phosphatidylinositol 3-kinase/Akt pathway in ET-1-mediated migration.
In this study, ET-1 induced contraction of PSCs through both ETA and ETB receptors as assessed by the contraction of collagen lattices. This is in agreement with the previous study showing that both ETA and ETB receptors play an important role in contraction of colonic myofibroblast cells[26], but disagrees with hepatic stellate cells where ET-1 induced contraction is through ETA receptor[14]. Investigation of cellular contraction using collagen lattices (gels) was introduced by Bell et al[39] and has been validated as a model of study of cellular contraction in a variety of cell types. Fibroblasts seeded on top of type I collagen gels bind and organize underlying collagen fibrils, both in close proximity and at a distance from cells[40]. After such reorganization, lattices are converted into a dense mass with the morphological appearance of dermal scar. Kawada et al[41] reported a morphological study of ET-1-induced contraction of hepatic stellate cells on hydrated collagen gels. They demonstrated that contracted form of the stellate cells was morphologically characterized by the retraction of dendritic processes and the bending of the extended cell bodies that enfold the underlying collagen matrix. Because the cell body and processes are both closely attached to the collagen fibers, their contraction generates effective forces for the contraction of collagen gels.
ET-1 did not alter the proliferation of PSCs. The effect of ET-1 on growth and the underlying mechanisms depend on cell type. For example, ET-1 promotes the proliferation of human pulmonary artery smooth muscle cells through ETA and ETB receptors[25]. ET-1 did not affect the proliferation of human colonic myofibroblast cells[26]. In human hepatic stellate cells, ET-1 has been shown to inhibit the serum-induced proliferation through ETB receptor, whereas ET-1 did not induce proliferation by itself[15]. Of note, Pinzani et al[14] reported that ET-1 induced proliferation of early-passaged human hepatic stellate cells (passages 1 and 2), but the effect was less evident at a later stage of culture. This phenomenon is attributable at least in part to the progressive shift from a relative predominance of ETA receptor to ETB receptor during culture[14]. Because the effects of ET-1 on cell growth might be mediated by distinct ET receptors, the ratio between ETA and ETB receptors in the cells might constitute an additional factor that could affect the sensitivity of cells to the growth regulatory effects of ET-1. We and others have shown that ERK is a key mediator of PDGF-BB-induced mitogenic signals in PSCs[16,42]. In spite of the ability to activate ERK, ET-1 failed to alter the proliferation, suggesting that activation of ERK is required but not sufficient for the proliferation of PSCs. This is also in the case of stimulation by ethanol, acetaldehyde, and 4-hydroxy-2,3-nonenal in PSCs[43], and in agreement with the concept that activation of ERK, although necessary, may not be sufficient to justify the mitogenic properties of an agonist[44].
Klonowski-Stumpe et al[45] reported that activated PSCs expressed ETA receptor and ET-1, and that ET-1 increased cytosolic Ca2+ concentration[45]. Exogenous ET-1 did not induce contraction, and, even in the absence of exogenous addition of ET-1, PSCs reduced the area of hydrated collagen lattices to 75% of the original value within 6 h, which was significantly attenuated by BQ-123, but not by another ETB receptor antagonist IRL-1 038. The reason for discrepancy of the results between our study and theirs remain unknown, but the different experimental systems (e.g., different cell passages, different ET-1 concentrations, different ETB receptor antagonist, and different serum-starvation condition) employed may be one explanation. As the ETA receptor antagonist inhibited basal contraction of PSCs in their study, the endogenous ET-1 from PSCs or ET-1 that remained in the culture supernatant appeared to be responsible for the basal contraction of PSCs. In addition to ETA receptor, we here have shown for the first time that activated PSCs express ETB receptor, and that exogenously added ET-1 induces migration of PSCs.
It has been accepted that hepatic stellate cell contraction in response to ET-1 plays an important role in the regulation of sinusoidal blood flow, and in the development of portal hypertension and hepatic fibrosis[12,13]. ET-1 is markedly overexpressed in different cellular elements in cirrhotic liver tissue, particularly in sinusoidal endothelial cells and activated hepatic stellate cells located in the sinusoids of the regenerating nodules at the edge of fibrous septa[16]. On the other hand, the pathophysiological implication of ET-1-induced contraction of PSCs remains obscure. Although PSCs are located pred-ominantly in the periacinar regions in normal pancreas[1,2] perivascular and periductal localization has been also reported Ikejiri[46]showed that vitamin A-storing cells (now recognized as PSCs) were perivascularly localized in the pancreas of rats fed with vitamin A. Perivascular PSCs might participate in the regulation of blood flow and the development of microvascular ischemia during pancreatitis through ET-1 production[47]. Izumi et al[48] reported that PSCs were found in the pancreatic duct wall of normal pancreas, and that the amount of such cells was excessively increased in patients with focal pancreatitis. They assumed that circular contraction of the ductal wall by proliferating PSCs might induce the stenosis of the main pancreatic duct and block the efflux of pancreatic juice from branch pancreatic ducts at the stenotic portion, leading to localized acinar atrophy and fibrosis[48]. In addition, PSCs might participate in control of the intraductal pressure, epithelial cell reconstitution, and duct remodeling during the healing of ductal injury. Along this line, it is now well accepted that the contractile activity of myofibroblasts is responsible for connective tissue remodeling during wound healing[38]. Obviously, further studies are necessary to clarify the pathop-hysiological relevances of ET-1-mediated cell functions of PSCs