Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.6018
Revised: March 20, 2005
Accepted: March 23, 2005
Published online: October 14, 2005
AIM: To evaluate serum alanine aminotransferase (ALT) activity in a well-characterized group of uncomplicated obese subjects and its correlation with insulin resistance, plasma adiponectin, and leptin concentrations.
METHODS: One hundred and five uncomplicated obese subjects (87 women, 18 men, age 34.3±9.6 years, BMI 39.9±8.3 kg/m2) were studied. Serum ALT activity was evaluated. Insulin sensitivity was assessed by euglycemic hyperinsulinemic clamp (M index) and fasting insulin. Plasma leptin and adiponectin levels were also measured.
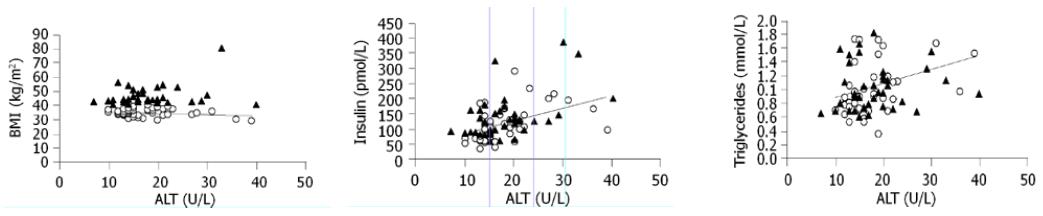
RESULTS: Serum ALT concentration in the whole group of uncomplicated obese subjects was 17.73±6.33 U/L with none of the subjects presenting ALT levels greater than 43 U/L and only 9 (11%) women and 3 (19%) men showed ALT levels >19 and >30 U/L for women and men, respectively. No significant difference was detected in serum ALT levels between severe obese subjects (BMI >40 kg/m2) and those with BMI <40 kg/m2 (18.63±6.25 vs 17.26±6.02 U/L). ALT was significantly correlated with fasting insulin (r = 0.485, P = 0.02) and triglycerides (r = 0.358, P = 0.03).
CONCLUSION: Serum ALT activity is practically normal in uncomplicated obese subjects, independently of their obesity degree. These findings suggest the role of obesity-related comorbidities and not of BMI as main risk factors for elevated ALT levels in obese subjects.
- Citation: Iacobellis G, Moschetta A, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Normal serum alanine aminotransferase activity in uncomplicated obesity. World J Gastroenterol 2005; 11(38): 6018-6021
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/6018.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.6018
Overweight and obesity have been reported to be major risk factors for elevated serum alanine aminotransferase (ALT) activity[1,2] and non-alcoholic fatty liver disease (NAFLD)[3-6]. High serum ALT levels have also been proposed as a marker of risk for type 2 diabetes[7,8]. A recent atherosclerosis study showed a significant relationship between ALT activity and insulin resistance[7]. In addition, unexplained ALT elevation has been found to be associated with increased visceral adiposity and other features of the metabolic syndrome[2]. Nevertheless, no studies evaluating serum ALT activity in metabolically healthy obese subjects have been performed. Uncomplicated obesity, as previously shown by our group[9] could be a good model to clarify the influence of obesity per se on ALT levels, without the confounding effect of the obesity-related comorbidities.
In this study, we sought to evaluate the serum ALT activity in a well-characterized uncomplicated obese subjects and its possible correlation with insulin resistance, plasma adiponectin, and leptin concentrations.
We selected 105 consecutive Caucasian uncomplicated obese subjects (body mass index [BMI] >30 kg/m2) from 600 obese subjects who were referred to our Day Hospital from Rome and surrounding areas between January 2001 and June 2004. The selected subjects had the following features: 87 women, 18 men; age, 34.3±9.6 years (range, 20-55 years); BMI, 39.9±8.3 kg/m2 (range, 30-80.1 kg/m2); duration of obesity, 15±5 years (range, 10-30 years). Uncomplicated obesity was defined according to the following parameters: no signs, symptoms and history of liver diseases (alcoholic hepatitis, viral hepatitis, positive serum hepatitis B surface antigen, positive serum hepatitis C surface antibody, auto-immune hepatitis, drug-induced hepatitis, familial metabolic disorders, history of fatty liver during pregnancy, portal hypertension, liver cancer, elevated serum transferrin saturation, >2 drinks of alcohol per day), no other clinically significant abnormalities on physical examination, no lipid-lowering, hypoglycemic or antihypertensive drugs, no history of cardiovascular and respiratory diseases, normal ECG, normal fasting glucose (<6.10 mmol/L), normal glucose tolerance (2 h glucose levels <7.7 mmol/L during oral glucose tolerance test [OGTT] systolic blood pressure <17.76 kPa and diastolic blood pressure <11.18 kPa for at least three measurements, normal plasma lipids (total cholesterol <5.18 mmol/L, high-density lipoprotein cholesterol [HDL-C] >1.03 mmol/L for men and >1.29 mmol/L for women, low-density lipoprotein cholesterol [LDL-C] <3.36 mmol/L and triglycerides <1.69 mmol/L). Subjects that met all these criteria were defined as uncomplicated obese subjects and included in the study.
This study was conducted in accordance with the guidelines proposed in the Declaration of Helsinki and approved by the review committee of La Sapienza University. All subjects gave their written informed consent before the study began.
Anthropometrical measurements Weight (to the nearest 0.1 kg) and height (to the nearest 0.5 cm) were measured while the subjects were fasting and wearing only their undergarments. BMI was calculated as body weight (kg) divided by height and squared (m2) and was used as a marker of obesity. Minimum waist circumference (cm, minimum circumference between thelower-rib margin and the iliac crest, midwaist) was measured while the subjects were standing with their heels together.
Clamp study We performed euglycemic hyperinsulinemic clamp according to previously described method[10] in all obese subjects, after 10-12 h overnight fast. A polyethylene cannula was inserted into an antecubital vein for the infusion. A second catheter was inserted into the antecubital vein controlaterally to determine plasma glucose and insulin concentrations. Insulin was continuously infused at the rate of 4.0 mU/kg per min for 5 min, 2.0 mU/kg per min for 5 min, and 1.0 mU/kg per min for 110 min. The plasma glucose concentration was measured every 5 min after the start of the insulin infusion and a variable infusion of 20% glucose was adjusted based on the negative feed back principle to maintain the plasma glucose levels at fasting plasma glucose with a coefficient of variation <5%. Plasma samples were collected every 20 min for determination of insulin concentrations. The steady state of the test was considered as the interval between 60 and 120 min. In these standard conditions, glucose infusion requirement in order to maintain euglycemia equals whole body insulin mediated glucose disposal, indicating the degree of insulin sensitivity. Whole body glucose utilization (M index) was calculated from the infusion rate of exogenous glucose during the second hour of the insulin clamp period after correction for changes were made in glucose levels in a distribution volume of 250 mL/kg. The M index was adjusted by kilograms of fat-free mass (FFM kg).
Impedensitometry measurements Fat mass (FM) and fat-free mass (FFM) were estimated using a bioelectrical impedance analyzer (BIA-103; Akern, Florence, Italy).
Analytical procedures Serum ALT activity was assayed using a Hitachi 737 analyzer (Boehringer Mannheim Diagnostics, IN, USA). Serum aspartate aminotransferase (AST) activity and g-glutamyltranspeptidase (gGT) were also measured. AST/ALT ratio was calculated. Plasma glucose was determined by the glucose oxidase method (mmol/L, reference range: 4.16-6.10; autoanalyzer, Beckman Coulter, Inc., Fullerton, CA, USA; [CV 1.9±0.2%]). Plasma insulin concentrations were determined by RIA kit (pmol/L, reference range: 34.5-173.6; Linco Research, Inc., St. Louis; CV, 3.0±0.3%). Blood samples for plasma insulin measurements were collected in heparinized tubes. Plasma leptin concentrations were determined by RIA kit (mg/L, reference range: 1.0-7.8; Linco Research, Inc., St. Louis, MO, USA; CV, 3.7±0.5%). To evaluate day-by-day plasma leptin variation, we measured plasma leptin concentrations at 24-h intervals in all subjects. Leptin concentrations had a very small interday variation (mean variation, 3.4±0.6%). Plasma adiponectin concentrations were also measured by RIA kit (reference range: 1.5-100 ng/mL; Linco Research, Inc., St. Louis, MO, USA; intra- and inter-assay CVs 4.5% and 3%, respectively). Samples were diluted 500 times before assay.
Data in the text and in the tables are expressed as mean±SD. Mann-Whitney test with 95%CI was applied to evaluate the differences between all parameters. Linear regression analysis was performed to identify correlates of ALT. Two-tailed P<0.05 indicated statistical significance. Analysis was done using Stata 5.0 (Stata Corp., College Station, TX, USA).
Anthropometric, clinical, and hormonal parameters of uncomplicated obese subjects are summarized in Table 1. A wide BMI range occurred in our uncomplicated obese subjects (30-80 kg/m2). Fifty-nine subjects had a BMI <40 kg/m2 and 46 subjects showed a BMI >40 kg/m2. No differences on age, glucose and lipid patterns and blood pressure between the two obese groups were found. Although women were prevalent in this cohort, no difference on sex distribution was observed.
| BMI <40(n=59) | BMI >40(n=46) | P | |
| Age (yr) | 36.4±9.2 | 32.8±8.5 | NS |
| Women (n) | 49 | 38 | NS |
| FFM (kg) | 57.9±7.4 | 56.3±7.35 | NS |
| Fasting glucose (mmol/L) | 4.69±0.47 | 4.77±0.44 | NS |
| 2 h OGTT glucose (mmol/L) | 5.90±1.08 | 6.18±1 | NS |
| Total cholesterol (mmol/L) | 4.84±0.67 | 4.61±0.63 | NS |
| HDL-C (mmol/L) | 1.36±0.3 | 1.30±0.28 | NS |
| LDL-C (mmol/L) | 3.17±0.64 | 3.23±0.62 | NS |
| Triglycerides (mmol/L) | 0.98±0.34 | 1.02±0.34 | NS |
| Systolic BP (kPa) | 16.05±1.31±3.4129.8 | 16.05±1.38 | NS |
| Diastolic BP (kPa) | 10.13±1.11 | 10.26±1.10 | NS |
| Fasting insulin (pmol/L) | 95.4±55.8 | 139.2±70.8 | <0.05 |
| Leptin (μg/L) | 27.4±16.4 | 45.5±17.9 | <0.01 |
| Leptin/BMI ratio | 0.76±0.30 | 0.86±0.35 | NS |
| Adiponectin (mg/L) | 40.3±21.4 | 25.20±17.3 | <0.05 |
| ALT (U/L) | 17.26±6.02 | 18.63±6.25 | NS |
| AST (U/L) | 24.65±9.82 | 29.46±10.82 | NS |
| AST/ALT ratio | 1.43±0.49 | 1.56±0.45 | NS |
| γGT (U/L) | 28.12±16.51 | 26.34±15.591 |
Serum ALT concentration in the whole group of uncomplicated obese subjects was 17.73±6.33 U/L with none of the subjects presenting ALT levels greater than 43 U/L. Only 9 (11%) uncomplicated obese women and 3 (19%) men showed ALT levels >19 and >30 U/L for women and men, respectively.
No significant difference was detected for serum ALT levels between severe obese subjects (BMI >40 kg/m2) and those with BMI <40 kg/m2 (18.63±6.25 U/L vs 17.26±6.02 U/L, respectively).
Overall, fasting insulin average level was 126.7±67.3 pmol/L (range 42.3-411.5 pmol/L). Plasma insulin concentration average achieved at the steady state of the euglycemic hyperinsulinemic clamp was 1 011.8±177 pmol/L. The whole body glucose utilization (M index) during the clamp was 8.9±4.1 mg/FFM kg per min. Coefficient of variation of blood glucose was less than 4% in each clamp study throughout the test. Severe obese subjects (BMI >40) had lower M index than obese subjects with BMI <40 kg/m2 (7.35±3.5 vs 9.43±3.78 mg/FFM kg per min, P<0.01, 95%CI 0.71-3.44).
Plasma leptin concentration in all obese subjects was 34.14±16.01 mg/L. Leptin adjusted for BMI (leptin/BMI ratio) was 0.84±0.33. As expected, severe obese subjects had higher plasma leptin levels than mild/intermediate obese subjects (P<0.01, 95%CI 11.4-24.7). No statistically significant difference on leptin/BMI ratio between the two groups was found (Table1).
Plasma adiponectin concentration in all obese subjects was 28.9±18.5 mg/L (range 5.9-90.6 mg/L). Severe obese subjects had lower plasma adiponectin levels than mild/intermediate obese subjects (P<0.01, 95%CI -23.5 to -7.1, Table 1).
Linear simple regression analysis showed that ALT was significantly correlated with fasting insulin (r = 0.485, P = 0.02) and triglycerides (r = 0.358, P = 0.03, Figure 1). No significant correlation existed between ALT and BMI, waist circumference, leptin, leptin/BMI ratio, adiponectin, M index, HDL-C, and total cholesterol. All the correlations were substantially unchanged stratifying for sex.
This study showed for the first time that serum ALT activity was normal in uncomplicated obese subjects, independently of their obesity degree. We have not found any correlation between ALT activity and BMI. Indeed, no significant difference in ALT activity between severe and mild/intermediate obese subjects was observed. Our data seem to be in disagreement with previous studies. In fact, it has been reported a significant relationship between BMI and serum ALT levels exists[1,2]. Moreover, unexplained aminotransferase elevation has been shown to be significantly associated with increasing BMI[11]. Nevertheless, no studies on uncomplicated obesity have been previously performed. The presence of obese subjects with no comorbidities explains the discrepancies with previous studies, even if overweight (BMI 25-30) has been reported to be correlated with elevated ALT more than obesity alone[1]. This study was performed on a well-characterized group of uncomplicated obese subjects that despite a large amount of FM and a wide BMI range showed normal ALT activity. This novel finding could suggest that the risk of elevated ALT activity and NAFLD is independent of BMI, if the obesity-related complications are not present. Indeed, uncomplicated obese subjects have a normal serum ALT activity and cardiovascular function, as we have previously shown[12].
We have found a significant, although relatively weak, correlation between fasting plasma insulin, triglycerides, and ALT activity. These data are of potential interest and consistent with previous studies showing a relationship between insulin resistance and ALT activity[7,8]. Although our uncomplicated obese subjects presented normal ALT levels, we could speculate that insulin-resistant obese subjects may have a higher risk to develop liver dysfunction and NAFLD. Insulin resistance could play a role in developing liver functional changes, more than obesity per se, similar to what we described for cardiovascular parameters[12]. At a variance of other studies[3], we have not found an association between plasma leptin concentration and serum ALT levels. Since plasma leptin and BMI are well-known to be strongly correlated, this could explain the lack of correlation between leptin and ALT in the present series of patients. Finally, waist circumference, previously shown to be associated with ALT activity, could be ineffective in predicting central adiposity if a wide range of BMI occurred.
Taking all the considerations together, the present study performed on a large number of subjects clearly suggests the absence of a direct role of a high BMI per se on perturbing ALT activity in obese subjects.
Nevertheless, we recognized that the present data should be considered with caution. We studied only serum ALT activity that cannot be used as a marker for histological diagnosis[13]. Therefore, our conclusions are based solely on liver enzymes. Even if ALT levels were not elevated in the majority of the uncomplicated obese subjects, as compared to stringent criteria of normality[14,15], we realized that no inference can be drawn on liver function from this study.
In conclusion, serum ALT activity is normal in uncomplicated obese subjects, independently of their obesity degree. These findings suggest that the role of obesity-related comorbidities and not of BMI is the main risk factors for elevated ALT levels in obese subjects.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 406] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 945] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 3. | Nakao K, Nakata K, Ohtsubo N, Maeda M, Moriuchi T, Ichikawa T, Hamasaki K, Kato Y, Eguchi K, Yukawa K. Association between nonalcoholic fatty liver, markers of obesity, and serum leptin level in young adults. Am J Gastroenterol. 2002;97:1796-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 646] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 5. | Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 6. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1725] [Cited by in RCA: 1742] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 7. | Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Kempf J, Zinman B, Haffner SM. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 503] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 9. | Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, Di Mario U, Leonetti F. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res. 2002;10:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214-E223. [PubMed] |
| 11. | Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol. 1999;94:3010-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Iacobellis G, Ribaudo MC, Zappaterreno A, Vecci E, Tiberti C, Di Mario U, Leonetti F. Relationship of insulin sensitivity and left ventricular mass in uncomplicated obesity. Obes Res. 2003;11:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 814] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 14. | Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1-407. [PubMed] |
| 15. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1049] [Article Influence: 45.6] [Reference Citation Analysis (4)] |