Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.5973
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: October 14, 2005
AIM: To evaluate the effects of gliadin on the oxidative environment in the “ in vivo-like ”model of a three-dimensional cell culture system.
METHODS: LoVo cell line (intestinal adenocarcinoma) multicellular spheroids were treated with digested gliadin (with albumin used as a control). Spheroid volumes, cell viability and morphology, lactate dehydrogenase (LDH) release, content of reduced glutathione (GSH) and activity of GSH-related enzymes were examined. The data were statistically analyzed using the Student’s t-test (P<0.05). was considered statistically significant.
RESULTS: Gliadin reduced cell viability (from 20% to 60%) and led to morphological alterations characterized by apoptotic findings and cytoskeletal injuries. LDH activity increased. The content of GSH reduced (-20% vs controls), and activity of GSH-related enzymes was significantly inhibited.
CONCLUSION: Gliadin treatment induces an imbalance in the antioxidative mechanism of cells cultured by the three-dimensional technique. This alteration may explain the cell damage directly caused by gliadin and the subsequent morphological abnormalities.
- Citation: Dolfini E, Elli L, Roncoroni L, Costa B, Colleoni MP, Lorusso V, Ramponi S, Braidotti P, Ferrero S, Falini ML, Bardella MT. Damaging effects of gliadin on three-dimensional cell culture model. World J Gastroenterol 2005; 11(38): 5973-5977
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/5973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.5973
Since Hudson et al[1] first observed the inhibited growth and morphological modifications in different human cell lines induced by gliadin exposure, a number of in vitro studies have confirmed the cytotoxicity of the peptide. Gliadin has agglutinating activity on K562 cells (human chronic myeloid leukemia), reduces F-actin content in intestine 407 cells, inhibits cell growth, induces apoptosis and alters redox equilibrium in Caco-2 cells (human intestinal adenocarcinoma), and causes a rearrangement of the cytoskeleton through the zonulin pathway and the loss of tight junction permeability in IEC-6 cells[2].
Three-dimensional cell cultures (multicellular tumor spheroids, MCTSs) were first introduced by Sutherland et al[3] in the early 1970s, and are now considered as an interesting model in biomedical research[4]. Unlike conventional monolayer cell culture systems, MCTSs maintain the specific morphological and biochemical properties of the corresponding in vivo tissue, and remain in a differentiated and functionally active state for many weeks, thus making it possible to study the long-term effects of various xenobiotics[5]. We have recently used MCTSs from the LoVo cell line to test gliadin, and have shown that it has a direct cytotoxic effect on cell growth and morphology[6].
Redox equilibrium plays a pivotal role in cell homeostasis, and can affect biological functions[7,8]. Glutathione (GSH), which consists of L-glutamine, L-cysteine and glycine, is the most important low-molecular-weight peptide involved in redox equilibrium. It has a reductive action on the cell environment and neutralizes the reactive oxygen compounds and free radicals formed during metabolism. GSH depletion induces low thiol protein stores, a determinant of cell homeostasis, and triggers cytotoxicity[9,10]. GSH depletion has also been shown in a number of human diseases (alcoholic liver disease, HIV infection, acute respiratory distress syndrome, and inflammatory bowel disease), and it has been shown that depressed intracellular GSH levels in liver and mammary tissues promote carcinogenesis[11].
The aim of this study was to evaluate the content of reduced GSH and the activity of GSH-related enzymes in LoVo MCTSs treated with gliadin.
Gliadin was purified from Triticum aestivum flour (Hereward cultivar) according to Capelli et al[12]. Bovine serum albumin (BSA) used as a control was purchased from Sigma (Milan, Italy). Pepsin was supplied by Sigma (Milan, Italy) and pancreatin by Merck (Milan, Italy). All the chemicals were of analytical grade. Digestion was performed as previously described[6]: briefly, gliadin was first incubated with pepsin at 37 °C for 24 h, and then with pancreatin at 37 °C for 3 h, adjusting to pH 8.0.
The digested proteins were analytically controlled by reverse-phase HPLC, size-exclusion HPLC and SDS-PAGE before being freeze-dried and stored.
LoVo human colon adenocarcinoma cell line (ATCC, Rockville, USA) was maintained in exponential monolayer growth and routinely checked for mycoplasma contamination as previously described[6].
MCTSs were initiated according to Dolfini et al[6] by seeding 2105 cells/mL in 25 mL of complete medium in polycarbonate Erlenmeyer flasks (Corning, Milan, Italy), and incubated in a gyratory rotation incubator. MCTS volumes were evaluated according to Chignola et al[13].
On the 7th d, MCTSs (mean±SD: 22070 mm) were exposed to digested gliadin (PT-gliadin, 500 mg/mL) or BSA (PT-BSA, 500 mg/mL) in completely renewed medium for further 5 d, and subsequently taken for the evaluation of lactate dehydrogenase (LDH) release, GSH content and GSH-related enzyme activity, and morphological analysis.
MCTS viability was tested (colony-forming assay) by plating a cell suspension obtained after trypsin disaggregation of MCTSs (on the 5th d of treatment at PT-gliadin concentrations of 125, 500, 750, and 1 000 mg/mL, and a PT-BSA concentration of 1 000 mg/mL) in triplicate in six-well plates (500 cells/well). The surviving cell fraction was calculated after 10 d and compared with the plating efficiency of the controls.
LDH released from damaged cells was measured in free aliquots of medium from the cell cultures, and cell-free complete medium was included as a negative control.
Briefly, 50 mL of the aliquots of cell supernatants was mixed with 25 mL of LDH reagent (Sigma, Milan, Italy) and incubated at room temperature for 30 min. LDH activity was calculated by measuring the increase in absorbance at 490 nm according to Legrand et al[14] and related to the protein content of MTCSs[15]. LDH activity was reported as percentages of control values.
MTCSs were washed with PBS (Sigma, Milan, Italy), and then sonicated and centrifuged. The cytosolic supernatant was used to measure GSH content and activity of the GSH-related enzymes, namely reductase (GSR), peroxidase (GPOX) and GSH-S-transferase (GST).
GSH content and enzyme activity were analyzed according to previously described methods[16] and expressed as percentages of control values.
LoVo cell line MCTS samples were prepared as previously described[6] fixed in 2.5% glutaraldehyde in a phosphate buffer, and then washed in the same buffer. In order to avoid injury or loss of spheroids, they were encapsulated in a solidifying agar solution and small spheroid-containing cubes were routinely processed for transmission electron microscopy (TEM)[17]. The semi-thin (0.5 mm) sections used for light microscopy analysis were stained with toluidine blue, the ultra-thin sections (50-70 nm) used for the ultrastructural study were counterstained with uranyl acetate and lead citrate. The MCTSs were studied for the presence of microvilli and intercellular junctions, and the appearance of nuclei, cytoplasm and intracytoplasmic organelles.
Scanning electron microscopy (SEM) was performed using a Philips mod. XL20 scanning electron microscope. MCTSs were washed twice with PBS, and then fixed in 2.5% glutaraldehyde in a phosphate buffer at 4 °C for a minimum of 24 h. At the time of analysis, a representative spheroid sample was recovered, immediately placed on a filter paper, and observed in low vacuum modality at a high voltage of 10 kV.
Each experiment was repeated four times. All the data were expressed as mean±SD and analyzed using the two-tailed Student's test. P<0.05 was considered statistically significant.
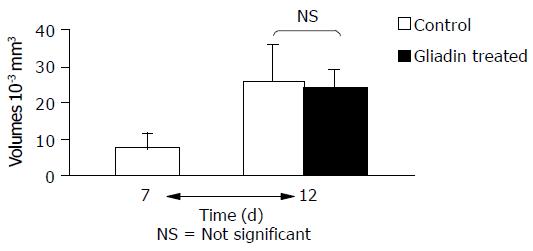
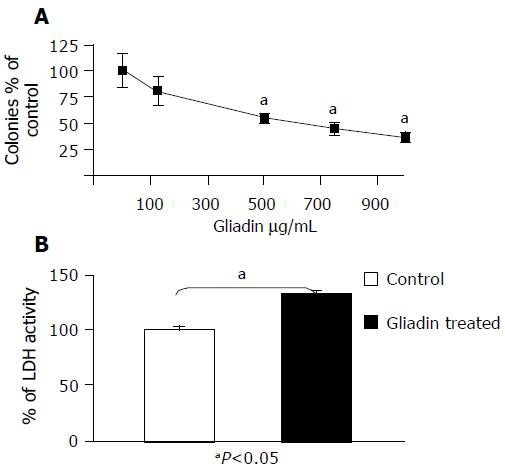
The untreated and control MCTSs (treated with PT-BSA) did not show any statistical difference in terms of diameter (27710-3 mm3vs 261010-3 mm3), viability (98% of the colonies formed by untreated MCTSs), LDH release (102% of the activity of untreated MCTSs), or microscopic appearance (data not shown). The volumes of MCTSs treated with PT-gliadin were similar to those of the untreated or control MCTSs (Figure 1), but their viability was 20-50% less than that of the controls and inversely related to PT-gliadin concentrations (Figure 2A). LDH activity in the medium of treated MCTSs was significantly increased by about 30% (Figure 2B).
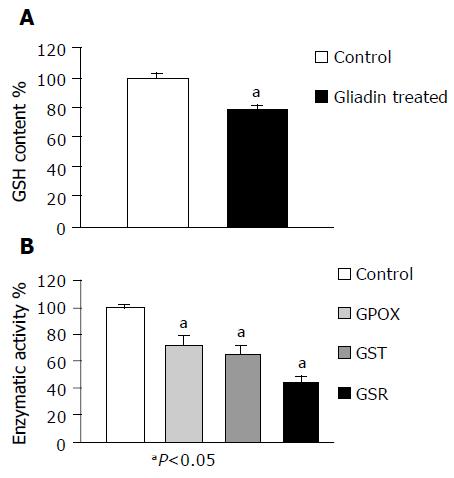
As shown in Figure 3A, the GSH content of treated MCTSs was about 20% less than that observed in the controls (P<0.05). The activity of GSH-related enzymes (GSR, GST, and GPOX) was also significantly decreased (by respectively 56%, 34%, and 27%, Figure 3B).
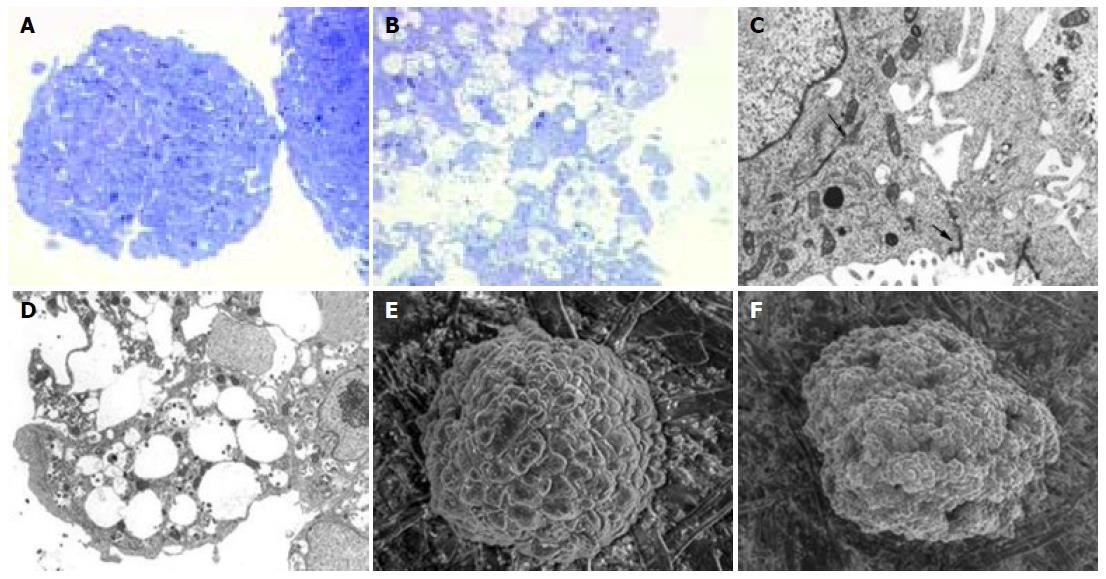
At light microscopy, both the treated and control MCTSs were spherical or oval in shape, but the control cells grew in a solid manner (with pseudoglandular differentiation, and without necrosis or apoptosis), whereas those in PT-gliadin-treated MCTSs showed nucleic displacement, a foamy cytoplasm and loss of cell adhesion: 20-50% had pycnotic nuclei and condensed chromatin (Figures 4A and B).
TEM showed that the control MCTSs had microvilli on their external surface and normal nuclei, cytoplasmatic organelles (rough endoplasmic reticulum, mitochondria, Golgi complex, and lysosomes), and cytokeratin tonofilaments. The cells were joined by complexes of tight, intermediate and desmosome junctions. In the treated MCTSs, microvilli disappeared from the cell surface and there were cytoskeletal and tight junction injuries. The cytoplasm contained electron-dense material in numerous phagosomes and frequent vacuoles of various sizes. Some cells showed cytoplasmatic lipid-like droplets and cannibalism (Figures 4C and D).
SEM of the control MCTSs revealed an ovoid or spherical shape with compact cells, which were densely organized and tightly packed together, but clearly distinguishable from each other. The treated MCTSs were irregular in shape with loosely packed cells, and the external surface was focally interrupted by irregularly distributed holes and blebs (Figures 4E, F, and 5).
Our findings show that gliadin had significant functional and morphological cytotoxic effects on intestinal adenocarcinoma cell line (LoVo) cultured in a three-dimensional model.
Gliadin is a protein characterized by a particularly high proline and glutamine content (respectively 15% and 35% of the residues) that forms a kink in the polypeptide structure that prevents peptidase attack[18].
Over the last few years, a number of studies based on the in vitro two-dimensional cell culture system have investigated the cellular effects of gliadin in order to clarify its role in the pathogenetic "Puzzle "of celiac disease (CD)[19]. Gliadin alters the cytoskeleton through the network of actin filaments, and damages tight junctions (TJ) by creating leaks in intercellular spaces, and these effects may be responsible for the loss of epithelial permeability and the changes in cell-cell signaling associated with CD[20]. Furthermore, gliadin also has a pro-apoptotic and agglutinating effect, inhibits cell growth and viability[2] and modifies the redox status of Caco-2 cells, thus causing a loss in reductive potential and an increase in the levels of reactive oxygen species[21].
Oxidative balance plays a pivotal role in cell homeostasis, and it has been suggested that its imbalance may be involved in various human diseases (liver diseases, HIV infection, pulmonary diseases, tumors, Parkinson'S disease, myocardial ischemia, and inflammatory bowel disease)[9,11]. GSH and its enzymatic machinery represent one of the most important cellular defenses against oxidative agents and harmful xenobiotics. Three enzymes are mainly involved in the GSH cycle: GPOX, which converts peroxides into less dangerous fatty acids, water, and GSH disulfide (GSSG); GSR, which reduces GSSG to GSH in NADPH-dependent reaction; and GST, which is involved in detoxification from xenobiotic compounds[22]. GSH has also been implicated in other cell functions: apoptosis, cell differentiation, prostaglandin synthesis, DNA repair, amino acid transport, enhanced immune response, and enzymatic activation[23].
The reduced GSH content and GSH-related enzyme activity observed in our gliadin-treated MCTSs confirm the dangerous effect of this peptide in an experimental model that maintains some of the biochemical and morphological features of the corresponding in vivo tissue[5], and support the hypothesis that relates a deficiency in an oxidable substrate[24]. The disturbed redox equilibrium is associated with the reduced cell viability in treated MCTSs and the alteration in the integrity of plasma membrane, as demonstrated by the leakage of large molecules such as LDH into the medium[25].
These functional alterations affect the morphology of MCTSs and their cells, whose clearly foamy cytoplasm and peripherally displaced nuclei, together with the fact that many are pycnotic with marginally condensed chromatin, support the presence of apoptotic processes[26]. TEM confirmed cell injury by revealing the presence of numerous phagosomes and frequent vacuoles of different sizes in the cytoplasm, as well as intra-cytoplasmic lipid-like droplets. The external ring normally consisting of columnar cells with serrated intercellular TJs and surface microvilli resembling normal enterocyte epithelia was altered in our treated MCTSs, which showed disrupted TJs and microvilli (Figure 4). SEM also showed that the treatment causes a loss of organization in cells contained in the external layer of spheroid, with the formation of hole-like structures and an abundant presence of apoptotic blebs[27].
Although our data cannot explain whether the redox imbalance is due to a resource-consuming mechanism, decreased nuclear expression or protein synthesis (an effect previously associated with gliadin exposure[28]), they clearly demonstrate that gliadin has a direct damaging effect on human intestinal cells. Furthermore, we have confirmed that the three-dimensional cell culture system is a good experimental model for investigating the effects of different peptides, and can therefore be added to the techniques used to study CD.
The authors would like to thank the "Centro per lo Studio della Celiachia, University of Milan, for its logistic support and Kevin Smart (LINK Srl, Milan) for his help in preparing the manuscript.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Hudson DA, Cornell HJ, Purdham DR, Rolles CJ. Non-specific cytotoxicity of wheat gliadin components towards cultured human cells. Lancet. 1976;1:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Elli L, Dolfini E, Bardella MT. Gliadin cytotoxicity and in vitro cell cultures. Toxicol Lett. 2003;146:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Sutherland RM, Inch WR, McCredie JA, Kruuv J. A multi-component radiation survival curve using an in vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Kunz-Schughart LA, Kreutz M, Knuechel R. Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int J Exp Pathol. 1998;79:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 236] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Kunz-Schughart LA. Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biol Int. 1999;23:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Dolfini E, Elli L, Ferrero S, Braidotti P, Roncoroni L, Dasdia T, Falini ML, Forlani F, Bardella MT. Bread wheat gliadin cytotoxicity: a new three-dimensional cell model. Scand J Clin Lab Invest. 2003;63:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 7. | Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 835] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 8. | Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 1685] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 9. | Burg D, Mulder GJ. Glutathione conjugates and their synthetic derivatives as inhibitors of glutathione-dependent enzymes involved in cancer and drug resistance. Drug Metab Rev. 2002;34:821-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res. 1988;202:343-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 298] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Lomaestro BM, Malone M. Glutathione in health and disease: pharmacotherapeutic issues. Ann Pharmacother. 1995;29:1263-1273. [PubMed] |
| 12. | Capelli L, Forlani F, Perini F, Guerrieri N, Cerletti P, Righetti PG. Wheat cultivar discrimination by capillary electrophoresis of gliadins in isoelectric buffers. Electrophoresis. 1998;19:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Chignola R, Schenetti A, Chiesa E, Foroni R, Sartoris S, Brendolan A, Tridente G, Andrighetto G, Liberati D. Oscillating growth patterns of multicellular tumour spheroids. Cell Prolif. 1999;32:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Legrand C, Bour JM, Jacob C, Capiaumont J, Martial A, Marc A, Wudtke M, Kretzmer G, Demangel C, Duval D. Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium [corrected]. J Biotechnol. 1992;25:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 309] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 16. | Dolfini E, Elli L, Dasdia T, Bufardeci B, Colleoni MP, Costa B, Floriani I, Falini ML, Guerrieri N, Forlani F. In vitro cytotoxic effect of bread wheat gliadin on the LoVo human adenocarcinoma cell line. Toxicol In Vitro. 2002;16:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Ryter A, Kellemberg E. Encapsulating methods for isolated cells. Fixation, dehydration and embedding of biological specimens, 3rd ed. Oxford: North Holland Publishing Company 1998; 95. |
| 18. | Dewar D, Pereira SP, Ciclitira PJ. The pathogenesis of coeliac disease. Int J Biochem Cell Biol. 2004;36:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Elli L, Dolfini E, Bardella MT. Direct gliadin cytotoxicity as a cofactor in the pathogenesis of celiac disease. Int Arch Allergy Immunol. 2004;134:88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Rivabene R, Mancini E, De Vincenzi M. In vitro cytotoxic effect of wheat gliadin-derived peptides on the Caco-2 intestinal cell line is associated with intracellular oxidative imbalance: implications for coeliac disease. Biochim Biophys Acta. 1999;1453:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1119] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 23. | Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 368] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Toborek M, Hennig B. Fatty acid-mediated effects on the glutathione redox cycle in cultured endothelial cells. Am J Clin Nutr. 1994;59:60-65. [PubMed] |
| 25. | Gissel H, Clausen T. Excitation-induced Ca2+ influx and skeletal muscle cell damage. Acta Physiol Scand. 2001;171:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4271] [Cited by in RCA: 4190] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 27. | Hentze H, Latta M, Künstle G, Dhakshinamoorthy S, Ng PY, Porter AG, Wendel A. Topoisomerase inhibitor camptothecin sensitizes mouse hepatocytes in vitro and in vivo to TNF-mediated apoptosis. Hepatology. 2004;39:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Giovannini C, Mancini E, De Vincenzi M. Inhibition of the cellular metabolism of Caco-2 cells by prolamin peptides from cereals toxic for coeliacs. Toxicol In Vitro. 1996;10:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |