Published online Oct 7, 2005. doi: 10.3748/wjg.v11.i37.5763
Revised: February 15, 2005
Accepted: February 18, 2005
Published online: October 7, 2005
AIM: To investigate the effect of N-(4-hydrophenyl) retinamide (4-HPR), the derivative of retinoic acid, on inhibition of migration, invasion, cell growth, and induction of apoptosis in hepatocellular carcinoma cells (HCCs) and malignant melanoma cells.
METHODS: 4-HPR was chemically synthesized. Cellular migration and invasion were assayed by Borden chamber experiment. Cell growth was assayed by MTT chromometry. Apoptosis effect was measured using Hoechst 32258 staining and flow cytometry. Gene transfection was performed with lipofectamine.
RESULTS: We observed that the migration of HCC and melanoma cells was significantly suppressed by 4-HPR and the migration cells were reduced to 585.03 (control 20127.2, P < 0.05, n = 4) in SMMC 7721-k3 HCC, and to 25425.04 (control 30230.1, P < 0.05, n = 4) in melanoma cells after 6-h incubation with 4-HPR. The invasion through reconstituted basement membrane was also significantly reduced by 4-HPR treatment to 11.23.3 in SMMC 7721-k3 HCC (control 2713.1), and to 24.33.2 in melanoma cells (control 67.510.1, P < 0.05, n = 3). Cell growth, especially in melanoma cells, was also significantly inhibited. Furthermore, 3 mmol/L of 4-HPR induced apoptosis in B16 melanoma cells (37.110.94%) more significantly than all-trans retinoic acid (P < 0.05), but it failed to induce apoptosis in SMMC 7721-k3 HCC. The mechanism for 4-HPR-induced apoptosis was not clear, but we observed that 4-HPR could regulate p27kip1, and overexpression of cerebroside sulfotransferase (CST) diminished the apoptosis induced by 4-HPR in melanoma cells.
CONCLUSION: 4-HPR is a potent inhibitor of HCC migration and inducer of melanoma cell apoptosis. CST and p27kip1expression might be associated with 4-HPR-induced apoptosis.
-
Citation: Wu XZ, Zhang L, Shi BZ, Hu P. Inhibitory effects of
N -(4-hydrophenyl) retinamide on liver cancer and malignant melanoma cells. World J Gastroenterol 2005; 11(37): 5763-5769 - URL: https://www.wjgnet.com/1007-9327/full/v11/i37/5763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i37.5763
The incidence of malignancy is increasing, especially hepatocellular carcinoma and malignant melanoma. Patients with these tumors usually have poor prognosis and short survival because of early metastasis, and also have the highest morbidity rate among all tumors[1-3]. The tumor cells of these diseases have early metastasis potential, which makes the leading cause of cancer-related death[4,5]. However, there is some lack of knowledge regarding the mechanism of tumor cell migration and metastasis, although some metastasis modes or general process have been suggested by previous study[6]. The mechanism by which one or several neoplastic cells escape from the primitive tumor to start the metastatic process is still a matter of discussion[7,8]. The neoplastic cells escape, migration, invasion of blood vessel basement membrane, and growth at new locations are all involved in the process and potential of metastasis. It is completely unclear that what kind of circumstances and conditions will produce the escape and that what situation will favor tumor cells migration or invasion, although the invasion is correlated with matrix metalloproteinases activity[9,10]. The patients situations get worse due to lack of effective therapy and prevention of tumor metastasis. Hence, it will be more important to find an alternative approach or therapeutic agent that can inhibit the growth or metastasis of HCC and malignant melanoma.
Synthetic derivative of retinoic acid holds a great promise in the treatment against tumors[11]. With an artificial modification of the structure, N-(4-hydrophenyl) retinamide (4-HPR) shows a different activity with retinoic acid and has apoptosis-inducing potential[12]. To investigate its anti-tumor effects on HCC and melanoma cells, we used the synthetic analog and compared 4-HPR with retinoic acid. This study is supposed to present the evidence of inhibition of HCC and melanoma cells by 4-HPR and provide the experimental base for further application of anti-tumor activities.
Agarose, propidium iodide (PI), p-nitrophenyl phosphate (pNPP), all-trans retinoic acid, and 3-(N-morpholino)propane-sulfonic acid (MOPS) were from Sigma-Aldrich. Bovine serum albumin (BSA, V) was from Roche. Culture medium RPMI 1640 and fetal bovine serum (FBS) were the product of GIBCO. Transfection reagent lipofectamine was purchased from Life Technologies. Matrigel was from BD Bioscience. Transwell (Borden Chamber), 24- and 96-well cluster plates were from Costar Corning Company. Arsenic (III) oxide was from the Pharmaceutical factory, Harbin Medical University. 4-HPR was synthesized by us by achieving recrystal with the purity of 99.95%. Mouse mAbs against p27kip1 and p21waf1 were from NeoMarker Co. O4 mAb against lactosyl sulfatide and galactosyl sulfate was from Chemicon. Bicinchonic acid (BCA) protein assay reagent kit was from Pierce.
F1/B16 melanoma cell was purchased from ATCC. The 7721-k3 cell was a subline[13] selected from SMMC-7721 cells for the high migration capability.
Cell migration assay was performed as described in our previous report[13]. The assay was performed in Transwell with an insert in 6.5 mm diameter, which has a polycarbonate filter at the bottom. The filter is a microporous membrane and has 8.0 mm sized pores in the membrane. In the chamber under the insert, 600 mL DMEM containing 5 mL/L FBS was added as an attractant for cell migration. In control, 5 mL/L BSA, instead of 5 mL/L FBS, was added, which had no attraction to cells. The cells were digested with 2.5 mmol/L EDTA, washed twice with serum-free medium, resuspended in serum-free DMEM, and adjusted to a concentration of 2105/mL. The cell suspension of 100 mL was added into the insert and incubated at 37 °C in 50 mL/L CO2 for 6 h. After gentle scraping, the adherent cells on the top of the filter by cotton in 30 g/L acetic acid, the cells in the bottom of the filter were then fixed in methanol for 30 min and stained with 5 g/L crystal violet for 5 min. All the cells in the bottom of the filter were counted and the average cell number of four independent assays represented cell migration capacity. In the control group containing 5 mL/L BSA under the filter, few cells migrated through the polycarbonate filter with an average of 2.500.02, whereas in the test group containing 5 mL/L FBS under the filter, 334.7024.57 (n = 3) cells migrated to the bottom of the filter. Therefore, in the following experiment, 5 mL/L FBS was used as chemoattractant in chemotaxis assay.
Tumor cell invasion assay was also conducted in Transwell, but the polycarbonate filter was precoated by 20 mg matrigel per insert. After incubation at 37 °C for 1-2 h, the gel was dried and formed a thin layer to reconstitute base membrane. Like the migration assay, 600 mL of DMEM containing 5 mL/L FBS was added to the well under the filter as chemoattractant. Cell suspension (100 mL) containing 1105 cells was added into the insert. To observe the inhibition of tumor cell invasion, 3 mmol/L 4-HPR was added into the cell suspension. In the control group, 3 mmol/L 4-HPR was replaced by same amount of ethanol. After incubation at 37 °C with 50 mL/L CO2 for 36 h, the top surface of the filter was gently scrapped by a cotton stick and then the filter was fixed by methanol for 30 min and stained by 5 g/L crystal violet for 5 min. Cells on the bottom surface of the filter were all counted.
Cells are prepared in medium at a concentration of 3104/mL and 0.1 mL of cell suspension was placed on the 96-well plate. After incubation, 10 mL of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution (5 mg/mL) was added to each well and incubated at 37 °C for further 2 h. Then all the media in the well was discarded and the plate was washed with PBS. The plate was inverted onto an absorbent diaper to pull off the remaining solution in each well. Then 0.1 mL of DMSO was added to each well and the plate was gently shaken for 15 min. Data were read at 570 nm with a reference wavelength of 630 nm.
Acid phosphatase assay was performed according to our previous report[14] and the activity correlated well with cell number and MTT assay. Briefly, 3103 cells were placed on each well of a 96-well cluster plate. In the test group, 3 mmol/L 4-HPR was added, whereas the same amount of ethanol was added in the control group. After 12-48 h of incubation, the plate was washed twice with PBS (pH 7.4) and 100 mL reaction buffer (0.1 mol/L sodium acetate pH 5.0, 1 g/L Triton X-100, 5 mmol/L pNPP) was added into each well. After incubation at 37 °C for 2 h, the reaction was stopped by adding 10 mL of 1 mol/L NaOH. The data were read at 405 nm wavelength.
Cells were cultured at an initial density of 2105 cells/mL. The test group cells were incubated with 3 mmol/L 4-HPR (control with ethanol) at 37 °C for 6, 18, and 24 h, followed by washing twice with PBS. Cell pellets were then resuspended in 1 mL of hypotonic PI working solution (50 mg/mL PI, 1 g/L sodium citrate, 1 g/L Triton X-100) and incubated at 4 °C overnight. On the 2nd day, the cells were analyzed by a FACScalibur machine. Each sample containing at least 20 000 cells was acquired and measured by CellQuest software. The data of cell cycle were analyzed by ModFit LT version 2.0 software, fitting apoptosis mode.
Cells attached onto a glass slide were treated with 3 mmol/L 4-HPR. After washing twice with PBS, the slide was stained by Hoechst 32 258 and examined under a fluorescent microscope to count the cells with condensed, shrunk, and crumpled apoptosis nucleus among 200 cells, which were examined.
Cell cultures, maintained for the indicated time periods, were washed twice with ice-cold PBS, lysed with lysate buffer (50 mmol/L Tris-HCl, pH 7.4; 2 g/L Triton X-100, 1 mmol/L EDTA, 1 mmol/L PMSF). After being sonicated in ice for 5 min, protein concentration was determined using the BCA assay. Equal amounts of protein extracts were loaded onto 100 g/L sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted onto PVDF membrane, probed with antibodies against p27kip1 and p21waf1, developed using the ECF Western blotting kit, and visualized in Kodak X-ray film.
A full-length of cerebroside sulfotransferase (CST) gene expression frame[15] was inserted into an expressional vector pCXN2 and linearized by a restriction enzyme SspI. The vectors were then transfected into HCC and B16 melanoma cells. Transfection was performed with lipofectamine following the protocol provided by the manufacturer. After 48 h of transfection, all cells were screened with 400 mg/mL G418, and the colonies in clonal growth were selected, among which nine colonies with CST and six colonies with the vector pCXN2 transfection (Mock) were achieved. All the transfectants were further screened by Northern blot to determine mRNA expression level and by immunology assay with specific mAb O4 to confirm the products of CST enzyme.
A total of 20 mg RNA extracted from all transfectant cells was kept in a solution containing 500 mL/L formamide, 60 g/L formaldehyde, 20 mmol/L MOPS (pH 7.0), denatured at 65 °C, and then loaded onto 10 g/L agarose. After electrophoresis, RNA was blotted onto a nylon membrane and hybridized with a digoxigenin-labeled probe, which was a RNA fragment synthesized from pSVK-hCST (XhoI) by T7 RNA polymerase in vitro. Digoxigenin-labeled nucleotide was incorporated into the probe during RNA synthesis. After hybridization at 68 °C overnight and washing, the membrane was then incubated with mAb Fab2 against digoxigenin. Bands on the membrane were detected by a DIG Detection kit according to the manufacturer’s instructions.
The data were expressed as mean±SD. Difference analysis was made by Student’s t-test. P < 0.05 was considered statistically significant.
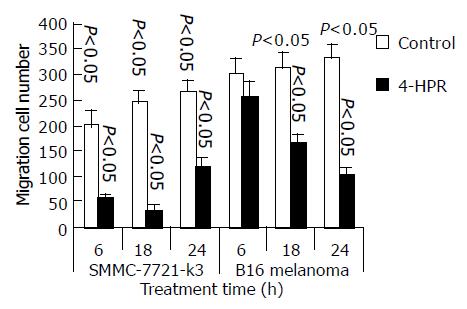
SMMC 7721-k3 HCC and B16 melanoma cells were both incubated at an initial concentration of 2105/mL with 3 mmol/L 4-HPR, which was dissolved in ethanol. The control cells were not incubated with the same amount of 0.1 g/L ethanol instead of 4-HPR. After 6-, 18-, or 24-h treatment, the cells were assayed for migration. The inhibitory effects of 4-HPR are shown in Figure 1. After a short period (6 h) treatment with 4-HPR, the migration ability of both cells was significantly inhibited and the migration rate in B16 melanoma cells declined to 84.1% of the control (Figure 1). After 18-h treatment with 4-HPR, the inhibitory effect became stronger. The migration rate after 24-h treatment got further down to 30.5% of the control. In HCC, the inhibitory effect was most significant after 18-h treatment (Figure 1).
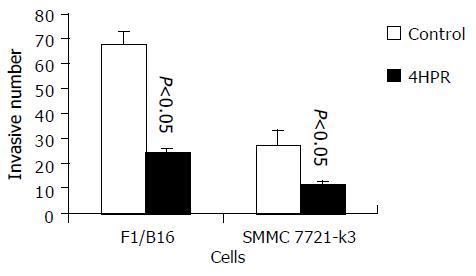
The invasion behavior of tumor cells is important in the metastasis process of hepatocellular carcinoma or melanoma cells. Tumor cell invasion ability can be evaluated and measured in vitro by means of the migration and penetration through a reconstituted base membrane. After 4-HPR treatment, 7721-k3 HCC and B16 melanoma cells were assayed for invasion capacity through the reconstituted base membrane (matrigel-coated chemotaxis filter). We observed a significant reduction of cells invading and migrating through the reconstituted base membrane in both SMMC 7721-k3 HCC and melanoma cells even after a short time period of 6-h incubation with 4-HPR. Melanoma cell invasion ability was inhibited more significantly and the cells invading and migrating through the membrane were reduced to 36% of the control. Moreover, invasion of HCC decreased to 41.4% of the control (Figure 2).
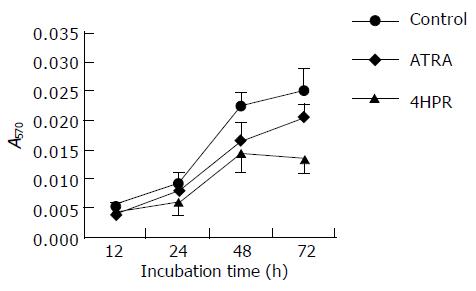
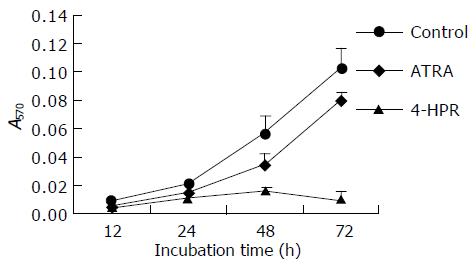
Cell growth was evaluated in vitro in a time-dependent manner. The treated cells were measured by MTT assay as well as acid phosphatase assay to indicate cell number after the treatment. HCCs seemed to be slightly resistant to low concentration (3 mmol/L) of retinoic acid treatment (ATRA). High concentration of ATRA (10 mmol/L) was needed for the inhibitory effect on the growth of SMMC-7721-k3 HCC. However, 4-HPR was able to inhibit SMMC-7721-k3 HCC growth at 3 mmol/L (Figure 3). After 72-h treatment, the inhibitory effect became significant as shown by the MTT assay (Figure 3). B16 melanoma cells were quite sensitive to 4-HPR treatment and the growth rate was significantly inhibited by 3 mmol/L 4-HPR. After 48-72 h treatment with 3 mmol/L 4-HPR, B16 melanoma cell growth was completely inhibited because the cell number did not increase, but decreased. The growth of B16 melanoma cells also decreased after treatment with 10 mmol/L ATRA, but the cell could still grow. The inhibitory effects of ATRA on B16 melanoma cell growth was not as significant as the inhibitory effects of 4-HPR (Figure 4).
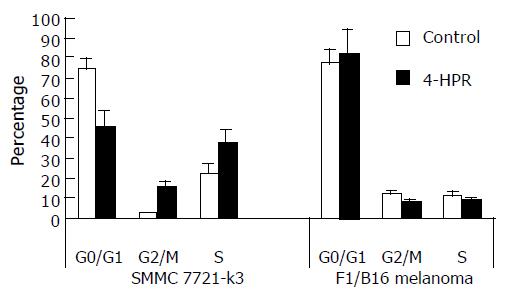
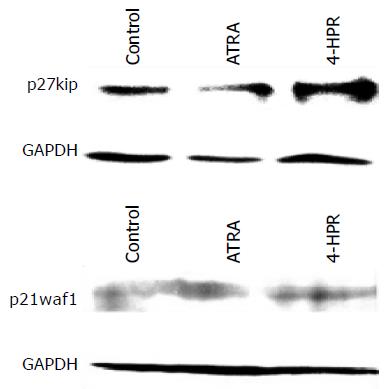
SMMC 7721-k3 HCCs were proliferated and the cell cycle was analyzed by flow cytometry. After 18-h treatment with 4-HPR, SMMC 7721-k3 HCCs at G0/G1 stage were significantly fewer, but at G2/M and S stage were significantly increased. After 24-h treatment with 4-HPR, cells at G2/M stage increased significantly (Figure 5), indicating that the cells were accumulated at this stage. However, cells at G0/G1 stage were the majority of the cells in control group, which accounted for 73.3%. Cells at S stage accounted for 22.95% and cells at G2/M stage were the minority (Figure 5). Total proteins extracted from the cells treated with ATRA or 4-HPR for 24 h was used to analyze p27kip1 and p21waf1 expression. We observed that 4-HPR treatment significantly induced p27kip1 expression, while p21waf1 retained the same expressional level after the treatment with either ATRA or 4-HPR (Figure 6). B16 melanoma cells were treated with 4-HPR for 18-24 h and the cycle did not change so much as SMMC 7721-k3 HCCs did. The percentage of different cell cycles remained at the similar level as control cells. However, 24 h after 4-HPR treatment, a peak of apoptosis appeared in B16 melanoma cells as detected by flow cytometry.
Apoptosis was evaluated using both Hoechst fluorescent staining and flow cytometry analysis by PI staining. SMMC 7721-k3 cells were not sensitive to 3 mmol/L 4-HPR induction and only few cells were induced into apoptosis. After 6-48 h treatment with 3 mmol/L 4-HPR, apoptotic cell population was less than 8% of all SMMC 7721-k3 cells, which was not significantly different from that in negative control. In positive control, the cells were treated with 3 mmol/L arsenic (III) oxide, 24.3% of SMMC 7721-k3 cells was induced into apoptosis, which was significantly different from that of negative control. B16 melanoma cells were treated with 3 mmol/L 4-HPR for 24 h and apoptotic cell population reached to 12.52% of the total cells. After 48-h treatment with 3 mmol/L 4-HPR, apoptotic cells accounted for 37.11% (Table 1). This indicated that 4-HPR might be a potent inducer of apoptosis for melanoma cells, but not HCCs. As for comparison, we observed the effect of ATRA on both SMMC 7721-k3 cells and B16 melanoma cells at the same time. No significant apoptosis could be detected after treatment with 3 mmol/L ATRA for 24-48 h, whereas about 20% apoptosis was observed in both hepatocellular carcinoma and melanoma cells after treatment with 20 mmol/L ATRA for 72 h (Table 1).
| Control | 4-HPR | ATRA | As2O3 | |
| 7721-k3 cells | 4.27 ± 0.06 | 8.53 ± 0.12 | 5.05 ± 0.03 | 24.27 ± 0.39 |
| P | > 0.05 | > 0.05 | < 0.05 | |
| B16 melanoma cells | 1.65 ± 0.16 | 37.11 ± 0.94 | 2.38 ± 0.38 | ND |
| P | < 0.05 | > 0.05 |
Our previous study demonstrated that retinoid compounds could inhibit cellular CST activity[16]; however, the inhibitory mechanism was unclear. Whether higher CST expression and activity would affect the action of retinoid compounds requires further investigation. In this study, we observed high CST expression influenced on 4-HPR induction in HCC and melanoma cells. After B16 melanoma and HCCs were transfected with whole expressional frame of CST cDNA in pCXN2 vector and CST mRNA expression was confirmed by Northern blot in the transfectants. All the cells were further screened using immune thin-layer chromatography with specific mAb against sulfolactosyl sulfatide and sulfogalactosyl sulfatide to select the clones with high expression of CST enzyme products. CST-transfected melanoma cell clones 1, 3 and CST-transfected HCC clone 6, 8 were chosen for the study. CST-transfected HCCs did not have any alteration in proliferation and cell growth, but the cell mobility, migration, and adhesion behaviors changed significantly, which led to promote metastasis of HCC in nude mice. The sensitivity to 4-HPR induction in CST-transfected HCCs remained the same as in vector-transfected cells (mock) or in parent HCC.
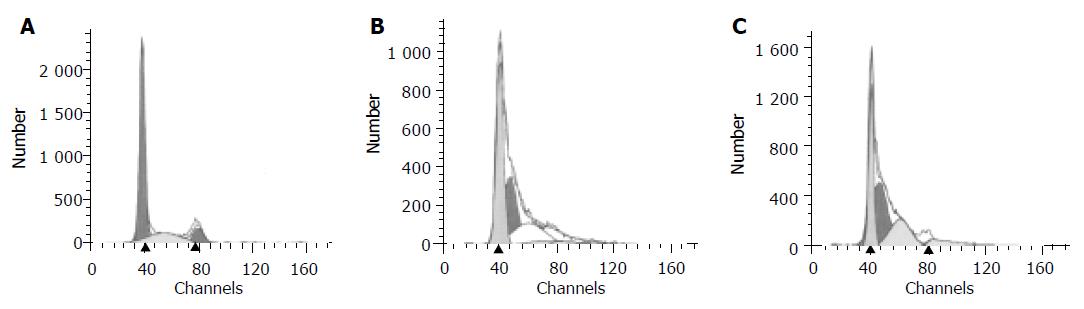
CST-transfected B16 melanoma cells, however, proliferated and grew in a faster rate than mock and parent B16 melanoma cells. Interestingly, CST-transfected B16 melanoma cells became less sensitive to 4-HPR induction than mock and parent melanoma cells. After treatment with 3 mmol/L 4-HPR for 48 h, 38.031.002% apoptotic cells were detected in mock transfectants. Moreover, after treatment with 3 mmol/L 4-HPR, parent B16 melanoma cells showed 37.110.95% apoptosis (Table 2). However, no significant difference of apoptotic rate was observed between the mock cells and parent melanoma cells (n = 4). The CST transfectant CST1 and CST3 of B16 melanoma cells became less sensitive to 4-HPR induction. After treatment with 3 mmol/L 4-HPR, there were only 32.651.98% and 33.590.91% apoptosis in CST1 and CST3 transfectants, respectively, which were significantly lower than those in mock cells (P < 0.05, n = 3). The results from flow cytometry analysis are similar to these. B16 melanoma cells were treated with 3 mmol/L 4-HPR and a peak of apoptosis appeared before peak G1(Figure 7). Mock cells had a similar peak of apoptosis as the parent cells. However, the size and height of apoptosis peak in both CST transfectants, CST1 and CST3, were much smaller or shorter than those of mock cells, which indicated that CST transfectant B16 melanoma cells produced fewer apoptosis cells after 4-HPR treatment. But, to ATRA the sensitivity remained the same in CST transfectants as those in mock cells.
Hepatocellular carcinoma is one of the most common malignant tumors. Up to now, there is no effective approach for treatment of the patients with HCC except surgical therapy. We have previously used all-trans retinoic acid to induce differentiation of HCCs and in clinical trials in some cases[17]. Although ATRA could induce HCCs differentiation and resulted in shrinkage of tumor mass in some patients, a higher dose of ATRA was required to achieve the effectiveness. The in vitroconcentration induction needed 10 mmol/L, which was 10 times higher than that for leukemia cells[18,19]. ATRA also was used for the induction of melanoma cells. Due to side effects, especially liver lesion, ATRA has a limited application for some patients. 4-HPR is a kind of synthetic retinoid derivative, which has been considered as the product with fewest side effects up to now. Several documents and clinical trials have suggested that 4-HPR is an effective agent for chemoprevention of breast cancer. Holven et al[20] reported that 4-HPR could inhibit liver secretion of retinoic acid binding protein and enhance the induction effect of retinoic acid.
In this study, we used the synthetic 4-HPR to observe the effect on hepatocellular carcinoma and melanoma cells. Quite different actions and effects were noted between 4-HPR and ATRA. To inhibit cell growth or migration of hepatocellular carcinoma and melanoma cells required relatively lower 4-HPR concentration as compared with ATRA. Our data showed that 3 mmol/L 4-HPR could significantly inhibit the migration of HCC and melanoma cells. After 6-h incubation with 3 mmol/L 4-HPR, the migration of B16 melanoma cells was inhibited to 84.1%. After 18-h incubation with 3 mmol/L 4-HPR, B16 melanoma cell migration was decreased to 52.4%. Similarly, the invasion ability of B16 melanoma cells through reconstituted basement membrane was significantly inhibited by 4-HPR treatment. Tumor cell migration and invasion were closely related with the metastasis behavior[21,22]. Thus, we consider that 4-HPR potentially inhibits the biological behaviors related with metastasis of melanoma cells and HCC and may have a potential application as the alternative treatment and prevention of tumor metastasis. In this study, we also observed the difference in effects between 4-HPR and ATRA. When B16 melanoma cells were treated with 3 mmol/L 4-HPR only for 24 h, a significant apoptosis rate was observed, no significant apoptosis could be detected after treatment with 3 mmol/L ATRA for 24 h. Induction of apoptosis in B16 melanoma cells or HCC needed a much higher concentration of ATRA and much longer incubation time (72 h) compared to 4-HPR. However, the mechanism of 4-HPR-induced apoptosis is not fully understood, although some lines of evidence have suggested the involvement of super oxygen reaction[23].
In our previous investigation[24], we observed that CST expression was closely associated with metastasis of tumor cells. When HCCs were inhibited by 10 mmol/L ATRA, the activity and products of cellular CST were significantly reduced. Overexpression of CST in HCC altered cell behaviors of migration through chemotaxis filters and adhesion to extracellular matrix. Overexpression of CST in melanoma cells promoted cell growth. Therefore, we observed 4-HPR induction, sensitivity, and other biologic behavioral changes in CST-expressing B16 melanoma cells. Interestingly, the induction of 4-HPR in such cells seemed to be less effective than in mock or parent cells because there were fewer apoptosis cells in CST highly expressing B16 melanoma cells than the mock cells. Although the possible mechanism is unclear, this result suggests that CST expression might be involved in 4-HPR-induced apoptosis in melanoma cells. Several studies have suggested that CST is closely related with some growth factors, such as HGF[25] and EGF[26,27], which stimulate cell growth. A recent report has suggested that HER2/neu can reduce the sensitivity of breast cancer cells to 4-HPR by activation of Akt, which further induces cyclooxygenase-2 (COX-2) expression[28]. Furthermore, 4-HPR has been recently reported to increase cellular ceramide levels, which is considered to be a cytotoxic messenger that may regulate the proliferation, survival, and death of cells[29,30]. The increased ceramide biosynthesis is required for 4-HPR-induced endothelial apoptosis[29]. Therefore, the metabolism or catabolism of cellular ceramide could affect the induction and sensitivity of 4-HPR. CST catalyzes sulfation of lactosylceramide or galactosylceramide[31], and thus promotes the metabolism of cellular ceramide.
We appreciate the National Nature Science Foundation of China (No. 30070183 and 30470398) for their support to this work.
Science Editor Guo SY and Kumar M Language Editor Elsevier HK
| 1. | Li X, Mikkelsen IM, Mortensen B, Winberg JO, Huseby NE. Butyrate reduces liver metastasis of rat colon carcinoma cells in vivo and resistance to oxidative stress in vitro. Clin Exp Metastasis. 2004;21:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Timmers M, Vekemans K, Vermijlen D, Asosingh K, Kuppen P, Bouwens L, Wisse E, Braet F. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer. 2004;112:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, Ohta T, Asaka M, Hirohashi S. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci. 2004;36:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Takamura M, Sakamoto M, Hirohashi S. Recent progress in study of mechanism of invasion and metastasis of hepatocellular carcinoma. Nihon Rinsho. 2001;59 Suppl 6:169-175. [PubMed] |
| 7. | Fiol J, Bolognese MG. Proto-metastatic core and comet effect: new theoretical model of the mechanism of metastasis generation. Med Hypotheses. 2000;54:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 8. | Xiong B, Yuan HY, Hu MB, Zhang F, Wei ZZ, Gong LL, Yang GL. Transforming growth factor-beta1 in invasion and metastasis in colorectal cancer. World J Gastroenterol. 2002;8:674-678. [PubMed] |
| 9. | Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Mönig SP, Baldus SE, Hennecken JK, Spiecker DB, Grass G, Schneider PM, Thiele J, Dienes HP, Hölscher AH. Expression of MMP-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2001;39:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Minton SE. Chemoprevention of breast cancer in the older patient. Hematol Oncol Clin North Am. 2000;14:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Clifford JL, Sabichi AL, Zou C, Yang X, Steele VE, Kelloff GJ, Lotan R, Lippman SM. Effects of novel phenylretinamides on cell growth and apoptosis in bladder cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:391-395. [PubMed] |
| 13. | Wu XZ, Chen YF. Fucosylated ogligosaccharides in the migration of hepatoma cells. Progress Biochem Biophys. 2002;29:932-937. |
| 14. | Lu H, Wu XZ. Acid phosphatase assay for measuring of the proliferation of tumor cells. Chin J Lab Med. 2001;24:84-86. |
| 15. | Hirahara Y, Tsuda M, Wada Y, Honke K. cDNA cloning, genomic cloning, and tissue-specific regulation of mouse cerebroside sulfotransferase. Eur J Biochem. 2000;267:1909-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Wu XZ, Li W, Ben J, Zha XL. Correlation between the expression level of sulfated lactosyl ceramide and metastasis potential of hepatocellular carcinoma. Progress Biochem Biophys. 2003;30:395-400. |
| 17. | Wu X, Lu H, Zhou L, Huang Y, Chen H. Changes of phosphatidylcholine-specific phospholipase C in hepatocarcinogenesis and in the proliferation and differentiation of rat liver cancer cells. Cell Biol Int. 1997;21:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Wu X, Wang X, Qien X, Liu H, Ying J, Yang Z, Yao H. Four years' experience with the treatment of all-trans retinoic acid in acute promyelocytic leukemia. Am J Hematol. 1993;43:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Wu X, Qureshi IA, Liu H, Yin J, Qian X, Ruijie X. Epidermal growth factor in acute promyelocytic leukemia treated with retinoic acid. Int J Hematol. 1995;62:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Holven KB, Natarajan V, Gundersen TE, Moskaug JO, Norum KR, Blomhoff R. Secretion of N-(4-hydroxyphenyl) retinamide-retinol-binding protein from liver parenchymal cells: evidence for reduced affinity of the complex for transthyretin. Int J Cancer. 1997;71:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Dellacasagrande J, Schreurs OJ, Hofgaard PO, Omholt H, Steinsvoll S, Schenck K, Bogen B, Dembic Z. Liver metastasis of cancer facilitated by chemokine receptor CCR6. Scand J Immunol. 2003;57:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Yamaguchi H, Kitayama J, Takuwa N, Arikawa K, Inoki I, Takehara K, Nagawa H, Takuwa Y. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J. 2003;374:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Goto H, Takahashi H, Fujii H, Ikuta K, Yokota S. N-(4-Hydroxyphenyl)retinamide (4-HPR) induces leukemia cell death via generation of reactive oxygen species. Int J Hematol. 2003;78:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Zhong Wu X, Honke K, Long Zhang Y, Liang Zha X, Taniguchi N. Lactosylsulfatide expression in hepatocellular carcinoma cells enhances cell adhesion to vitronectin and intrahepatic metastasis in nude mice. Int J Cancer. 2004;110:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Kobayashi T, Honke K, Miyazaki T, Matsumoto K, Nakamura T, Ishizuka I, Makita A. Hepatocyte growth factor specifically binds to sulfoglycolipids. J Biol Chem. 1994;269:9817-9821. [PubMed] |
| 26. | Kobayashi T, Honke K, Gasa S, Kato N, Miyazaki T, Makita A. Epidermal growth factor elevates the activity levels of glycolipid sulfotransferases in renal-cell-carcinoma cells. Int J Cancer. 1993;55:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Tsuda M, Egashira M, Niikawa N, Wada Y, Honke K. Cancer-associated alternative usage of multiple promoters of human GalCer sulfotransferase gene. Eur J Biochem. 2000;267:2672-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Simeone AM, Li YJ, Broemeling LD, Johnson MM, Tuna M, Tari AM. Cyclooxygenase-2 is essential for HER2/neu to suppress N- (4-hydroxyphenyl)retinamide apoptotic effects in breast cancer cells. Cancer Res. 2004;64:1224-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Erdreich-Epstein A, Tran LB, Bowman NN, Wang H, Cabot MC, Durden DL, Vlckova J, Reynolds CP, Stins MF, Groshen S. Ceramide signaling in fenretinide-induced endothelial cell apoptosis. J Biol Chem. 2002;277:49531-49537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Kabayama K, Ito N, Honke K, Igarashi Y, Inokuchi J. Suppression of integrin expression and tumorigenicity by sulfation of lactosylceramide in 3LL Lewis lung carcinoma cells. J Biol Chem. 2001;276:26777-26783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |