Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5742
Revised: January 2, 2005
Accepted: January 5, 2005
Published online: September 28, 2005
Hirschsprung’s disease (HD) is a disorder associated with congenital malformation of the enteric nervous system with segmental aganglionosis. Prevailing therapy includes a resection of the affected part of the bowel. However, patients often do not obtain complete functional improvement after surgical treatment. We present the case of a 25-year-old woman who had surgical treatment of HD in early childhood. After that procedure she had clinical features of constipation for years in the end, passing of stool once a week, requiring laxatives and enemas. We diagnosed an incomplete resection of the aganglionic bowel via rectal biopsy and resected the remaining aganglionic segment. Two months after surgery the patient’s bowel function improved to a frequency of 1-4 stools per day. We conclude that regular follow-up is required to identify HD patients with persistent alterations of bowel function after surgery. In patients presenting with constipation, recognition of a remaining aganglionic segment or other alterations of the enteric nervous system should be aimed at in an early stage.
- Citation: Werner CR, Stoltenburg-Didinger G, Weidemann H, Benckert C, Schmidtmann M, Voort IRVD, Andresen V, Klapp BF, Neuhaus P, Wiedenmann B, Mönnikes H. Megacolon in adulthood after surgical treatment of Hirschsprung’s disease in early childhood. World J Gastroenterol 2005; 11(36): 5742-5745
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5742.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5742
Hirschsprung’s disease (HD) is a birth defect affecting the bowel with segmental aganglionosis of the distal part of the gut due to disturbances in the development and migration of neural crest- derived cells which comprise the enteric nervous system. This dysfunction leads to constipation and, in the worst case, to megacolon congenitum. HD occurs in one of 5 000 live births and has a male predominance of about 4:1[1,13]. Most patients are diagnosed in early childhood and treated by resection of the involved part of the colon. However, patients often do not obtain complete functional recovery after surgical treatment[2]. Studies on the therapeutic outcome in HD have so far mainly focused on the efficacy of different surgical techniques[3-6]. We present a case underscoring the necessity of appropriate diagnostic and therapeutic procedures in patients with HD where alterations of bowel function persist even after surgery.
A twenty-five year old woman, who suffered from constipation for years, was referred to our out patient clinic by her family physician. Symptoms worsened after an episode of diarrhea eight months ago. Currently she passed stool only once a week with the help of enemas.
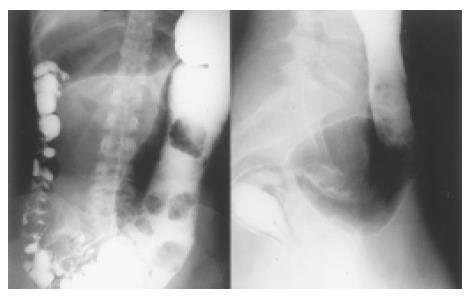
A colon contrast-medium enema showed a dilated colon proximal to a stricture of the rectum, located at about 8 cm proximal of the anus forming a megacolon (Figure 1). The dilated section of the colon reached mid-transverse colon, proximally followed by colon of normal filling pattern.
Three months after birth the patient underwent abdominal surgery because of clinical features of acute constipation suggesting HD. A two-step procedure was performed with colostomy and sigmoideostomy three months after birth. Four months later a definitive surgical solution was intended by resection of the aganglionic bowel and reanastomosis of the proximal normal bowel to the normal anal canal after Rehbein[7]. Resection of 16 cm gut was performed, including a constricted distal part and a dilated proximal segment with a coprolith in it. The complete excision of aganglionic bowel was confirmed by a pathologist. At the age of nine years, a complete obstruction of the small intestine occurred, and therefore resection of 9 cm of jejunum and removal of adhesions were required. During surgery a dilated hypertrophic descending colon was observed. After this surgical procedure, colonoscopy was performed with removal of tissue samples from the site of anastomosis of colon and anal canal. The colonoscopist reported no dilatation of the bowel, differing from the surgeon’s report. In contrast, the pathologist diagnosed aganglionosis in gut biopsies from aboral of the anastomosis. There was no enhanced activity of acetylcholinesterase in the segment proximal to the anastomosis. However, the patient was discharged with no definitive treatment for her constipation.
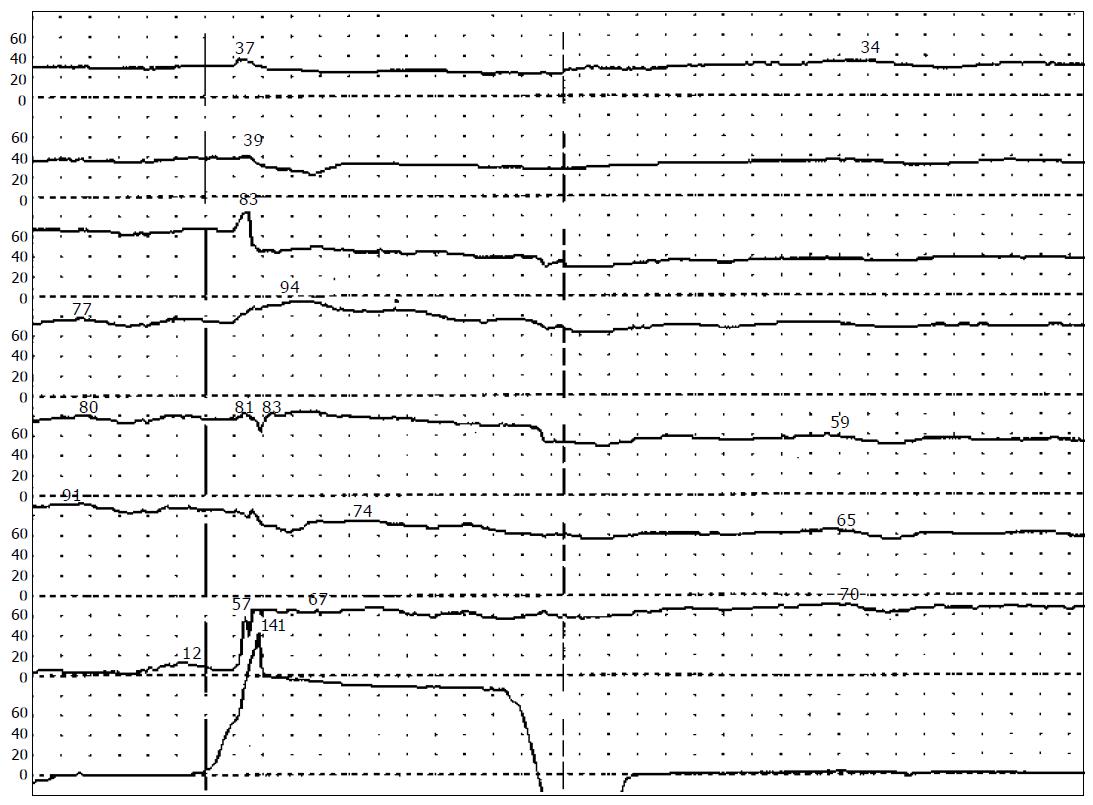
At initial presentation in our out-patient clinic, physical examination of the 25-year old patient with moderate adipose revealed surgical scars at the left lower abdomen and a longish palpable mass in the left abdomen reaching from left lower up to the left upper quadrant. Initial laboratory tests were nor mal with exception of hypercholesterinaemia. Anorectal manometry displayed absence of the anorectal inhibitory reflex at inflation of the balloon in the distal rectum (Figure 2).
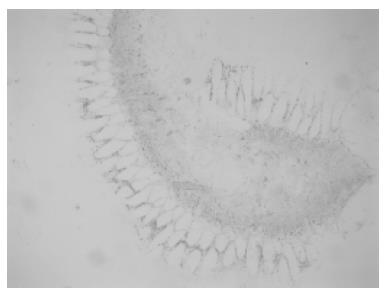
Rectal biopsies were taken and showed increased activity of acetylcholinesterase (Figure 3) in mucosal and submucosal layers and no ganglia of the plexus submucosus. Re-examination of slides of the specimen from the initial surgery 24 years ago revealed aganglionosis of both distal and proximal transection margins of the resected segment and extremely thickened nerve fibers replacing plexus myentericus.
We therefore concluded that at the abdominal surgery 24 years ago, removal of the entire aganglionic bowel was missed and an overseen aganglionic segment remained in situ. To remove the megacolon (Figure 4) and establish normal transit of stool through the bowel, we referred the patient to the surgical department where resection of the aganglionic segment with end-to-end anastomosis was performed. Complete resection of the aganglionic segment was confirmed via instantaneous sections during surgery (Figure 5).
A 51 cm long segment of the distal colon was resected up to that point in the more proximal colon where the instantaneous sections showed a physiological account of ganglia.
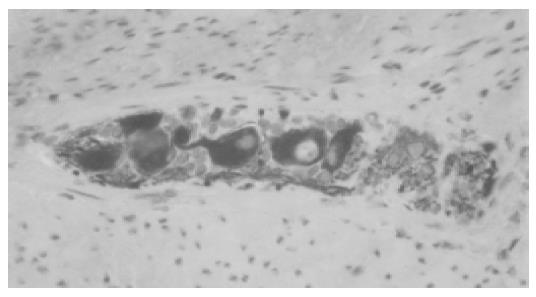
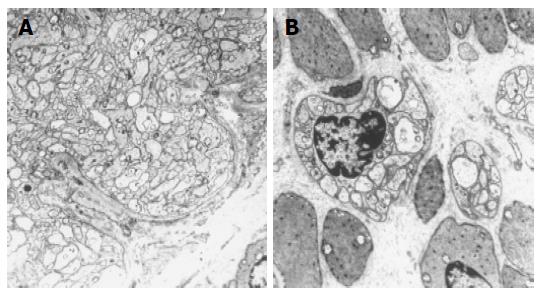
After surgery, microscopic examination of the mucosal, submucosal and muscular layers showed presence of a hypoganglionic segment in the distal part of the resected segment (Figure 6A) and presumptive secondary aganglionosis in the mid-part of the specimen. At the proximal transection margin, a number of normal-sized ganglia were present (Figure 6B).
The patient had a rapid recovery from surgery and was discharged after a few days. Upon re-examination two months after the surgery, the patient reported an excellent functional improvement. She passed stool regularly 1-4 times a day without the use of laxatives.
Newborns who fail to pass meconium within the first 48 h should be suspected of having Hirschsprung’s disease (HD) as one of the possible differential diagnoses[1]. However, if HD is not recognized at an early stage, it can present later in one or more of the following ways: delayed passage of meconium, complete intestinal obstruction (sometimes associated with a megacolon congenitum), and enterocolitis.
If diagnosis is confirmed by contrast enema, anorectal manometry and, as gold standard, a rectal biopsy with increased activity of acetylcholinesterase and absence of the plexus submucosus, sufficient resection has to be done, as the only successful treatment for HD is the resection of the aganglionic part of the bowel, including a dilated segment proximal to it. For definitive surgical treatment, several procedures have been developed[6-10].
In studies to elicit the long-term follow-up situation in patients who underwent surgical treatment of HD as infants[2-5], depending on the performed procedure, about 50% of patients report occasional complaints such as fecal soiling (43.5%), abdominal pain (34.8%), constipation (14.4%).
Constipation is a frequent finding among patients who underwent surgical treatment for HD (10.4%[3] to 14.4%[2]). Except for common reasons for constipation in adults, one has to consider additional differential diagnoses in patients who have had a surgical procedure because of HD in early childhood. Firstly, an incomplete resection of the aganglionic segment proximal to the anastomosis has to be ruled out. Secondly, a “transition zone”, i.e. a congenital hypoganglionosis or hyperganglionosis adjacent to the aganglionic segment[11] that causes constipative symptoms due to malfunction of the enteric nervous systems of this segment of the bowel has also to be ruled out. Thirdly, an intestinal neuronal dysplasia type B (INDB) has to be considered. This disease shows slowed intestinal transit time and an intractable constipation. Though aganglionosis is never present in INDB, there may be an increase of extrinsic nerve fibers in the affected gut as in HD[12,13] which could be mistaken for HD by rectal biopsy. Furthermore, HD often includes a region of INDB-like characteristic proximal to the aganglionic segment[11].
Another possibility is the secondary involution of the enteric nervous system and fibrosis of the bowel wall due to recurrent infections, causing features of constipation.
Depending on the diagnosis and if normal bowel function is not sustainable by conservative therapy, surgical treatment should be considered, as it was in our patient. Especially in young patients a long- lasting dependency on strong laxatives and enemas means a significant reduction in quality of life.
In Hirschsprung’s disease, regular follow-up examinations are required to identify patients with persisting alterations of bowel function after surgery. In HD patients with consatipation or other symptoms of impaired bowel movement after surgery, remaining of an aganglionic segment should be considered in differential diagnosis. Identification of this and other alterations of the enteric nervous system mentioned above should already be aimed at in an early stage, in order to prevent secondary harms, such as development of a megacolon, as it happened in our patient.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Milla PJ. Hirschsprung disease and other neuropathies. In: Rudolph AM, Hoffman JIE., Rudolph CD, eds. Rudolph's pediatrics. Stamford Appleton & Lange 1996; 1115-1118. |
| 2. | Skába R, Simsová M. Long-term results and quality of life in patients after surgery in childhood for Hirschsprung's disease by the Kasai method of colo(ileo)rectoplasty. Rozhl Chir. 2002;81:622-627. [PubMed] |
| 3. | Bjornland K, Diseth TH, Emblem R. Long-term functional, manometric, and endosonographic evaluation of patients operated upon with the Duhamel technique. Pediatr Surg Int. 1998;13:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Hsu WM, Chen CC. Clinical and manometric evaluation of postoperative fecal soiling in patients with Hirschsprung's disease. J Formos Med Assoc. 1999;98:410-414. [PubMed] |
| 5. | Heikkinen M, Rintala R, Luukkonen P. Long-term anal sphincter performance after surgery for Hirschsprung's disease. J Pediatr Surg. 1997;32:1443-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | REHBEIN F. [Operative therapy of Hirschsprung's disease]. Langenbecks Arch Klin Chir Ver Dtsch Z Chir. 1953;276:540-543. [PubMed] |
| 7. | Swenson O, BILL AH. Resection of rectum and rectosigmoid with preservation of the sphincter for benign spastic lesions producing megacolon; an experimental study. Surgery. 1948;24:212-220. [PubMed] |
| 8. | Duhamel B. A new operation for the treatment of Hirschsprung's disease. Arch Dis Child. 1960;35:38-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 141] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Soave F. A New surgical technique for treatment of hirschsprung's disease. Surgery. 1964;56:1007-1014. [PubMed] |
| 10. | Boley SJ. New modification of the surgical treatment of hirschsprung's disease. Surgery. 1964;56:1015-1017. [PubMed] |
| 11. | Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 1. Pediatr Dev Pathol. 2002;5:224-247. [PubMed] |
| 12. | Meier-Ruge WA, Brönnimann PB, Gambazzi F, Schmid PC, Schmidt CP, Stoss F. Histopathological criteria for intestinal neuronal dysplasia of the submucosal plexus (type B). Virchows Arch. 1995;426:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Wiedenmann B, Riedel C, John M, Ahnert-Hilger G, Stoltenburg G, Waldschmidt J, von Deimling K, Riecken EO, Schier F. Qualitative and quantitative analysis of synapses in Hirschsprung's disease. Pediatr Surg Int. 1998;13:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |