Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5685
Revised: April 1, 2005
Accepted: April 2, 2005
Published online: September 28, 2005
AIM: To evaluate the efficacy of combination chemotherapy with interferon-α (IFN α ) and 5-fluorouracil (5-FU) in patients with advanced hepatocellular carcinoma (HCC).
METHODS: Twenty-eight HCC patients in advanced stage were enrolled in the study. They were treated with IFNα / 5-FU combination chemotherapy. One cycle of therapy lasted for 4 wk. IFNα (3×106 units) was subcutaneously injected thrice weekly on days 1, 3, and 5 for 3 wk, and 5-FU (500 mg/d) was administered via the proper hepatic artery for 5 consecutive days per week for 3 wk. No drugs were administered during the 4th wk. The effect of combination chemotherapy was evaluated in each patient after every cycle based on the reduction of tumor volume.
RESULTS: After the 1st cycle of therapy, 16 patients showed a partial response (PR, 57.1%) but none showed a complete response (CR, 0%). At the end of therapy, the number of patients who showed a CR, PR, or no response (NR) was 1, 10, and 17, respectively. The response rate for therapy (CR+PR) was 21.5%. Biochemical tests before therapy were compared between responsive (CR+PR) and non-responsive (NR) patients, but no significant differences were found for any of the parameters examined, indicating that no reasonable predictors could be identified in our analysis.
CONCLUSION: Attempts should be made to discriminate between responders and non-responders by evaluating tumor size after the first cycle of IFNα /5-FU combination chemotherapy. For non-responders, therapy should not proceed to the next cycle, and instead, different combination of anticancer drugs should be explored.
- Citation: Enjoji M, Morizono S, Kotoh K, Kohjima M, Miyagi Y, Yoshimoto T, Nakamuta M. Re-evaluation of antitumor effects of combination chemotherapy with interferon-α and 5-fluorouracil for advanced hepatocellular carcinoma. World J Gastroenterol 2005; 11(36): 5685-5687
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5685
Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide. Therapies for HCC have been developed, such as percutaneous ethanol injection therapy (PEIT), microwave coagulation therapy (MC), and radiofrequency ablation (RFA). For patients in whom these localized therapies cannot be used, transarterial chemoembolization (TACE) and liver transplantation may be selected. However, in cases with repeated recurrence, vascular invasion or distant metastasis were used; these methods often become ineffective or impossible, and the last treatment option is chemotherapy. Although it is thought that HCCs are generally resistant to anticancer drugs, combination chemotherapy has been used to increase the responsiveness of cancer cells.
Combination therapy with interferon-α (IFNα ) and 5-fluorouracil (5-FU) was first reported for the treatment of patients with colorectal cancer[1]. Recent studies indicated that the combined chemotherapy involving arterial injection of 5-FU and subcutaneous injection of IFNα was also markedly effective in some patients with advanced HCC. In these studies, the response rates to therapy were 62.5%[2] and 53.1%[3]. In the present study, we describe the use of this combination therapy in HCC patients for whom there were no other treatment options.
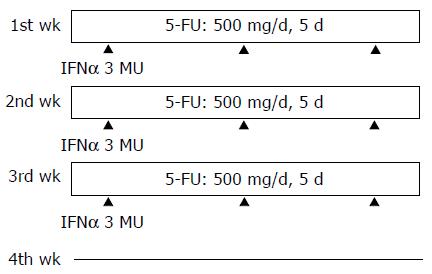
Between 2003 and 2004, 28 patients with unresectable HCC associated with multiple intrahepatic tumors and/or portal vein tumor thrombi received IFNα /5-FU combination therapy at the Third Department of Internal Medicine, Kyushu University Hospital. The treatment regimen is shown in Figure 1. IFNα (3×106 units) was subcutaneously injected thrice per week on d 1, 3, and 5 for 3 wk. 5-FU (500 mg/d) was given over 2 h through a catheter introduced into the proper hepatic artery for 5 consecutive days per week for 3 wk. No drugs were administered during the 4th wk. One cycle of the therapy lasted for 4 wk and patients received 1 -4 cycles. The effect of combination chemotherapy was evaluated in each patient after every cycle based on the reduction of tumor volume. The degree of the response was categorized as follows: (1) complete response (CR); depletion of all the tumors on computed tomography (CT); (2) partial response (PR); over 15% reduction of the total diameters of all detectable tumors on CT; and (3) no response (NR); those that did not show a CR or PR. Combination chemotherapy was stopped after the fourth cycle was completed, or when there was no reduction in the tumor volume after any cycle. At that time, final evaluations were performed.
The baseline characteristics of the patients who received IFNα /5 -FU combination chemotherapy are shown in Table 1. Because of the advanced stage of HCC in these patients, no other therapeutic options were available. Seven of the patients were being treated for HCC for the first time. Other therapies, such as surgical resection, PEIT, MC, RFA, and TACE, had been repeated at least twice in the other patients. Portal thrombi existed in Vp3 -4 in seven patients. The combination chemotherapy was performed on these patients (see Patients and methods).
| Patient (n) | 28 |
| Male/female | 21/7 |
| Portal thrombi (+/–) | 7/21 |
| TNM classification (II/III/IV) | 5/11/12 |
| Albumin | 3.4±0.1 (g/dL) |
| Total bilirubin | 1.3±0.2 (mg/dL) |
| ALT | 48.2±6.4 (IU/L) |
| Platelet | 11.3±1.7 (×104/mL) |
| PT | 70.9±2.8 (%) |
| AFP | 7 105±2 756 (ng/mL) |
| PIVKA-II | 11 922±4 274 (U/L) |
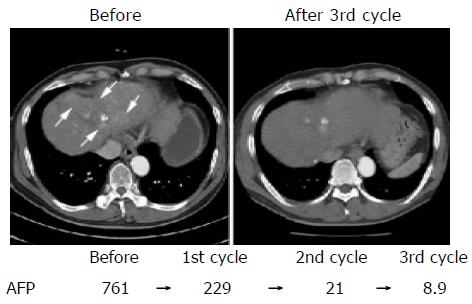
The response to chemotherapy is shown in Table 2. In these patients, chemotherapy was not stopped by adverse effects, such as thrombocytopenia and leukocytopenia. After the first cycle of therapy, 16 patients showed a PR (57.1%) but none showed a CR. In all the patients with PR, levels of the tumor marker α -fetoprotein (AFP) decreased (-2.6-91.2%, mean: -55.6%). When the therapy was completed, 1 of these 16 PR patients achieved a CR, and 10 of them showed a NR. The overall response rate for chemotherapy (CR+PR) was 21.5% (6/28). CT of a patient who achieved a CR is shown in Figure 2. After three cycles of therapy, the enhanced area of the HCC observed before chemotherapy disappeared and elevated levels of AFP decreased to the normal range (from 761.0 to 8.9 ng/mL). The backgrounds of the patients, including their laboratory test profiles before chemotherapy, were compared between responders (CR+PR) and non-responders; however, no significant differences were found for any of the parameters examined.
| Evaluation after 1st cyclepatient n (%) | Final resultspatient n (%) | |
| CR | 1 (3.6) | |
| PR | 16 (57.1) | 5 (17.9) |
| NR | 12 (42.9) | 22 (78.6) |
The mechanism underlying the ability of IFNα to strengthen the anti-cancer effect of 5-FU has been studied previously. IFNα enhances the conversion of 5-FU to an active metabolite, fluorodeoxyuridine monophosphate (FdUMP), through an increase of thymidine phosphorylase and a suppression of dihydropyrimidine dehydrogenase[4,5]. FdUMP induces DNA damage and the expression of death receptors (tumor necrosis factor-related apoptosis-inducing ligand [TRAIL] receptors) on cancer cells, resulting in cell death[6]. IFNα reduces tumor cell proliferation through cell cycle elongation[7] and induces p53, which contributes to tumor suppression[8]. IFNα also exerts immunomodulatory effects by stimulating T cells, NK cells, and monocytes[9,10], and enhances production of TRAIL by immune cells[11-13].
Several studies have attempted to distinguish between responders and non-responders to 5-FU/IFNα combination chemotherapy. Low levels (<50 ng/mL) of serum AFP were reported as a predictor[14], but conflicting results have also been reported[15]. A relationship between the expression levels of the IFN receptor on HCC cells and sensitivity to 5-FU/IFN therapy was reported previously[7], but another study group indicated that the expression levels of the type I IFN receptor were similar between responders and non-responders[3]. Yamamoto et al[6] reported that, among HCC patients who received 5-FU/IFNα combination chemotherapy, the expression of TRAIL mRNA in peripheral blood mononuclear cells (PBMC) was significantly higher in clinical responders than in non -responders. It was suggested that upregulation of TRAIL mRNA in PBMC may be a predictor of the clinical response to combination therapy. In our patients, no predictors were found among the biochemical tests that were analyzed. We advocate that, in practice, responders and non- responders should be discriminated after the first cycle of the therapy by evaluating tumor size and the levels of tumor markers. For non-responders, therapy should not proceed to the next cycle and instead a different therapeutic option should be explored.
Some groups have reported much better results from combination chemotherapy than those observed in our study[2,3]. Apart from the dose of IFNα used, their regimens were nearly identical to ours. Those studies used 5×106 units of IFNα per treatment. We previously tested the effect of IFNα at doses of 3×106 and 6×106 units, and found no significant difference (data not shown). We therefore used the lower dose of 3×106 units to decrease the incidence of adverse effects. Since other types of therapies have been repeated in many of our patients, the HCCs in these patients may have existed in polyclonal nodules. In fact, in some patients, some HCC nodules were reduced in size, and others were enlarged during the chemotherapy. It is possible that the polyclonality weakened the clinical effects of therapy. In our patients, a relatively high response rate, 57.1%, was observed after the first cycle, but resulted in a rate of 21.5% at the end of the last cycle. Many of the patients who initially showed a PR after the first stage ended up having NR at the end of therapy. These findings suggest that the original clones with resistance against anticancer drugs and some cancer cells that acquire resistance during therapy can proliferate, and their rate of growth increases synergistically whenever a cycle is repeated.
What can be done to make combination chemotherapy more effective? A better regimen for IFNα /5-FU combination chemotherapy could be developed. Which is better, IFNα or IFNβ ? IFN can be administered intravenously or intra- arterially. How many times should IFN be administered? What should be the dose of 5-FU at a time? What will be the best duration for 5-FU injection? It is also a problem whether the couple of 5-FU and IFNα is the best combination as chemotherapy for HCC. Many anticancer drugs can be selectable such as CDDP, MTX, LCV, and Gemcitabine. We should develop better regimen and combination for HCC.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Wadler S, Schwartz EL, Goldman M, Lyver A, Rader M, Zimmerman M, Itri L, Weinberg V, Wiernik PH. Fluorouracil and recombinant alfa-2a-interferon: an active regimen against advanced colorectal carcinoma. J Clin Oncol. 1989;7:1769-1775. [PubMed] |
| 2. | Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S, Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Moriyama M, Hoshida Y, Kato N, Otsuka M, Yoshida H, Kawabe T, Omata M. Genes associated with human hepatocellular carcinoma cell chemosensitivity to 5-fluorouracil plus interferon-alpha combination chemotherapy. Int J Oncol. 2004;25:1279-1287. [PubMed] |
| 4. | Schwartz EL, Baptiste N, Wadler S, Makower D. Thymidine phosphorylase mediates the sensitivity of human colon carcinoma cells to 5-fluorouracil. J Biol Chem. 1995;270:19073-19077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Dou J, Iwashita Y, Sasaki A, Kai S, Hirano S, Ohta M, Kitano S. Consensus interferon enhances the anti-proliferative effect of 5-fluorouracil on human hepatoma cells via downregulation of dihydropyrimidine dehydrogenase expression. Liver Int. 2005;25:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Yamamoto T, Nagano H, Sakon M, Wada H, Eguchi H, Kondo M, Damdinsuren B, Ota H, Nakamura M, Wada H. Partial contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway to antitumor effects of interferon-alpha/5-fluorouracil against Hepatocellular Carcinoma. Clin Cancer Res. 2004;10:7884-7895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Eguchi H, Nagano H, Yamamoto H, Miyamoto A, Kondo M, Dono K, Nakamori S, Umeshita K, Sakon M, Monden M. Augmentation of antitumor activity of 5-fluorouracil by interferon alpha is associated with up-regulation of p27Kip1 in human hepatocellular carcinoma cells. Clin Cancer Res. 2000;6:2881-2890. [PubMed] |
| 8. | Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 685] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 9. | Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71:565-581. [PubMed] |
| 10. | Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3048] [Cited by in RCA: 3079] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 11. | Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 373] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, Yagita H, Okumura K, Tanaka N, Taniguchi T. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol. 2001;31:3138-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Patt YZ, Yoffe B, Charnsangavej C, Pazdur R, Fischer H, Cleary K, Roh M, Smith R, Noonan CA, Levin B. Low serum alpha-fetoprotein level in patients with hepatocellular carcinoma as a predictor of response to 5-FU and interferon-alpha-2b. Cancer. 1993;72:2574-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Kurokawa Y, Matoba R, Nagano H, Sakon M, Takemasa I, Nakamori S, Dono K, Umeshita K, Ueno N, Ishii S. Molecular prediction of response to 5-fluorouracil and interferon-alpha combination chemotherapy in advanced hepatocellular carcinoma. Clin Cancer Res. 2004;10:6029-6038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |