Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5677
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: September 28, 2005
AIM: To evaluate the role of nitric oxide (NO) in the motor disorders of the dilated uninflamed mid-colon (DUMC) from trinitrobenzene sulfonic acid (TNBS)-induced acute distal colitis in rats.
METHODS: Colitis was induced in male Sprague-Dawley rats by a single intracolonic administration of TNBS. Control rats received an enema of 0.9% saline. The rats were killed 48 h after TNBS or saline administration. Macroscopic and histologic lesions of the colon were evaluated. Myeloperoxidase (MPO) and nitric oxide synthase (NOS) activity were measured on the colonic tissue. In TNBS rats, we evaluated spontaneous and evoked contractile activity in circular muscle strips derived from DUMC in comparison to the same colonic segment of control rats, both in the presence and in the absence of a non-selective NOS isoforms inhibitor N-nitro-L-arginine (L-NNA). Pharmacological characterization of electric field stimulation (EFS)-evoked contractile responses was also performed.
RESULTS: In TNBS rats, the distal colon showed severe histological lesions and a high MPO activity, while the DUMC exhibited normal histology and MPO activity. Constitutive NOS activity was similar in TNBS and control rats, whereas inducible NOS activity was significantly increased only in the injured distal colon of TNBS rats. Isometrically recorded mechanical activity of circular muscle strips from DUMC of TNBS rats showed a marked reduction of the force and frequency of spontaneous contractions compared to controls, as well as of the contractile responses to a contracting stimulus. In the presence of L-NNA, the contractile activity and responses displayed a significantly greater enhancement compared to controls. The pharmacological characterization of EFS contractile responses showed that a cooperative-like interaction between cholinergic muscarinic and tachykinergic neurokinin 1 and 2 receptors mediated transmission in DUMC of TNBS rats vs a simple additive interaction in controls.
CONCLUSION: The results of this study show that, during TNBS-induced acute distal colitis, circular muscle intrinsic contractile mechanisms and possible enteric neural excitatory activity are inhibited in the distended uninflamed mid-colon. Suppression of NO synthesis markedly improves spontaneous and evokes muscle contractions, in spite of any evident change in local NO activity.
- Citation: Onori L, Aggio A, D’Alo’ S, Muzi P, Cifone MG, Mellillo G, Ciccocioppo R, Taddei G, Frieri G, Latella G. Role of nitric oxide in the impairment of circular muscle contractility of distended, uninflamed mid-colon in TNBS-induced acute distal colitis in rats. World J Gastroenterol 2005; 11(36): 5677-5684
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5677.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5677
Changes in motility patterns have been described in the gastrointestinal tract in association with inflammatory conditions, independent of the etiologic origin[1,2]. Indeed, ulcerative colitis (UC), an idiopathic inflammatory disease of the colon, is associated with impaired intestinal motility such as defective colonic contractility[3-5] sometimes leading to dramatic colonic dilatation (toxic megacolon, TMC)[1]. Data derived from human colitis and from animal models of colitis suggest a clear relationship between the impaired colonic motility and the abnormal local production or release of inflammatory mediators and neuropeptides[6,7].
However, changes in motility patterns are not related only to the inflamed site of the colon[8-10]. In fact, in UC, concomitant distension of the small bowel and, less frequently, of the stomach has been reported[11,12]. Small bowel distension may even precede colonic dilation, and this has been termed as impending megacolon. This condition characterizes a subgroup of UC patients with a poor response to medical treatment and an increased risk for TMC, a need for surgery and mortality[11-14]. Furthermore, experimental colitis leads to changes in myenteric nerve function at inflamed and uninflamed gastrointestinal sites in the rat[15,16]. Several mechanisms have been hypothesized to explain motility disorders of an inflamed colonic segment[12,17,18].
In the last few years, growing evidence appears to suggest that nitric oxide (NO), synthesized by constitutive NO synthase (cNOS) expressed by certain descending interneurons in the myenteric plexus and by almost all enteric inhibitory non-adrenergic non-cholinergic motorneurons (iNANC), play a major role in the neuronal inhibitory control of intestinal mechanical activity in physiological Conditions[19-21]. NO exerts its action both through direct inhibition of smooth muscle contractility and negative modulation in myenteric excitatory motorneurons activity, mainly releasing acetylcholine and tachykinins[22]. Furthermore, in human beings and experimental severe colitis, there is evidence that a burst of NO production by inducible NO synthase (iNOS) in inflammatory and smooth muscle cells is responsible for severe motility disorders of the inflamed colon[23,24]. In fact, several studies have suggested that NO may be involved in the pathogenesis of acute colonic distension in severe UC and in animal model of acute colitis[25,26]. Nevertheless, little is known about the role of NO on motility disorders in intestinal tracts far from the inflamed segment[16].
Aim of the present study was to establish whether, in trinitrobenzene sulfonic acid (TNBS)-induced acute distal colitis in rats, abnormal NO activity was also responsible for the colonic motor disorders of the dilated mid-colonic segment apparently devoid of inflammatory alterations. In TNBS distal colitis, we, therefore, evaluated spontaneous and evoked contractions in circular muscle strips derived from the dilated uninflamed mid -colon (DUMC) and compared findings with those in the same colonic segment of control rats, both in the presence and absence of N-nitro-L-arginine (L-NNA), a non-selective nitric oxide synthase (NOS) isoforms inhibitor. Furthermore, NOS activity was also evaluated on samples of tissues adjacent to those used for the evaluation of mechanical activity.
Adult male Sprague -Dawley rats (250-300 g) were kept in a restricted access room at a constant temperature of 21-23°C with light/dark cycles of 12 h. The animals were housed in rack-mounted wire cages and fed with standard chow pellets and water ad libitum.
Adhering to the Italian National Research Council criteria for the care and use of laboratory animals, colitis was induced as originally described by Morris[27]. Briefly, colitis was induced in 10 rats under light ether anesthesia by a single intracolonic administration of 0.25 mL of 50% ethanol containing 30 mg of TNBS. The solution was introduced via a catheter placed 8 cm from the anal verge. Ten rats in the control group were given an enema of the same volume of 0.9% saline instead of TNBS. The rats were anesthetized and then killed by decapitation 48 h after TNBS or saline administration.
Laparotomy was performed under ether anesthesia, the colon was visualized, and the perimeter of the mid-colon was measured. Under anesthesia the entire colon was then rapidly excised and placed in a Petri capsule containing Tyrode solution (6.9 g/L NaCl, 0.35 g/L KCl, 0.16 g/L KH2PO 4, 0.29 g/L MgSO4, 1 g/L glucose, 0.28 g/L CaCl2, 2.1 g/L NaHCO3) continuously oxygenated with a mixture of 95% O 2 and 5% CO2 and maintained at a temperature of 37°C. The colon was carefully opened along the mesenteric side and the luminal surface was flushed with pre-warmed Tyrode solution through a fine needle syringe to remove the feces. If perforation of the organ was observed, the rat was excluded from the study. Fragments from the mid-colon (corresponding to the dilated non-inflamed region of the colon in TNBS rats) and the distal colon in both treated and control rats were processed for structural and enzymatic activity (myeloperoxidase (MPO), NOS). The mid- colon, from TNBS and control rats, was used for motility studies.
All tissue fragments were immediately and rapidly examined under a stereomicroscope by a blinded expert observer, and any visible damage was scored according to the following scale: 0 = no damage, 1 = localized hyperemia without ulcers, 2 = linear ulcers without inflammation, 3 = linear ulcers plus inflammation, 4 = two or more sites of ulceration and/or inflammation, 5 = ulceration >1 cm extension.
Samples from the DUMC and from the inflamed distal colon of TNBS rats and from mid- and distal colon of control rats were taken for conventional histology and for MPO activity evaluation.
For histology, tissue specimens were fixed in formalin and embedded in paraffin. Coded sections were then stained with hematoxylin and eosin, and examined at light microscopy, by a blinded observer.
For the measurement of MPO activity, full-thickness tissue, obtained from the sites adjacent to the tissue retrieved for histological studies, was snap frozen in liquid nitrogen and stored at -80°C. The MPO activity was measured according to the protocol of Wallace[28]. One unit of MPO activity was defined as the quantity of enzyme needed to convert 1 mol of hydrogen peroxide to water in 1 min, at room temperature, and was expressed in units per milligram of tissue.
Full thickness samples, obtained from DUMC and inflamed distal colon of TNBS rats and from mid- and distal colon of control rats, were used for the assessment of NOS activity.
NOS activity was determined by measuring the conversion of [3H]-L-arginine to [3H]-L-citrulline. Tissue samples were homogenized by sonication in homogenization ice-cold buffer (25 mmol/L Tris-HCl, pH 7.4, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 1 mmol/L ethylene glycol-bis ( β -aminoethyl ether) -N,N,N’,N’-tetraacetic acid containing protease inhibitors (0.2 mmol/L phenylmeth-anesulfonyl fluoride, 10 g/mL aprotinin, 10 g/mL pepstatin A, 10 g/mL leupeptin). The homogenates were centrifuged at 4°C for 20 min at 15 000 g. The supernatants were passed through AG50WX-8 Dowex resin (Bio-Rad Laboratories, Melville, NY, USA) to remove endogenous arginine. The protein content was determined by the bicinchoninic acid procedure (Pierce) using BSA as standard. Enzymatic reactions were conducted at 37°C for 30 min in 50 mmol/L Tris-HCl, pH 7.4, containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, 6 mol/L tetrahydrobiopterin (BH4), 2 mol/L flavin adenine dinucleotide, 2 mol/L flavin adenine mononucleotide, 1 mmol/L L-citrulline (to inhibit the catabolism of [3H]-L-citrulline), 60 mmol/L L -valine, 60 mmol/L L-ornithine, 60 mmol/L L-lysine (to inhibit nonspecific arginase activity) and 10 Ci/mL of [3H]-L-arginine (specific activity 66 Ci/mmol/L (Amersham, Buckinghamshire, UK). When indicated, CaCl2 (0.6 mmol/L) and calmodulin (CAM, 0.1 mol/L) were added to the assay system. This reaction was stopped by the addition of 0.4 mL of stop buffer (50 mmol/L Hepes, pH 5.5, 5 mmol/L EDTA). Citrulline was determined by introducing the samples into columns containing 0.1 mL of Dowex AG50WX-8, equilibrated resin to Na+ form, to bind all the unreacted radio-labeled L- arginine. The columns were vortexed and centrifuged (15 000 g for 30 s). Therefore, 100 µL of the eluate were counted by liquid scintillation to quantitate the formation of [3H]-L-citrulline. This assay measures both the calcium-dependent (constitutive) and the calcium-independent (inducible) isoenzymes. Any activity detected in the absence of Ca/CAM represented the iNOS activity. Quantitative results for citrulline-production are expressed as picomoles of citrulline per milligram proteins per minute (citrulline pmol/mg protein/min).
Tissue preparation Full wall thickness strips containing the mucosa (1.5 mm×10 mm) were derived both from the DUMC of the TNBS rats and the corresponding mid-colon of the control rats. Strips were oriented in the axis of the circular muscle and were attached to an isometric force transducer (Model Gemini Basile, Varese, Italy) and placed in an organ bath containing 20 mL of Tyrode solution which was continuously oxygenated with a mixture of 95% O2 and 5% CO2 and maintained at a temperature of 37°C. Muscle strips were allowed to stabilize for 2 h before they were stretched by 2-mm increments to the optimal length for contraction (Lo). The force of muscle contraction was normalized for cross-sectional area obtained by the following equation: XS = M/Lo ×d[29,30] , where XS (cm2)=cross-sectional area of muscle, M (g) = mass of circular muscle layer (after removal of the mucosa and submucosa), Lo (cm)=optimal length for muscle contraction, d (g/mL) = density of colonic muscle in bath numerically equal to specific gravity of colonic muscle in Tyrode solution. The muscle strips from TNBS rats which were devoid of spontaneous activity were, nevertheless, used to study the effects of NOS inhibitor (L- NNA) when basal activity was absent. These muscle strips, after 2 h of equilibration, were stretched to 1.25× resting length. In this way, each muscle strip was stretched to a similar length.
Spontaneous contractile activity The parameters considered were the frequency and force of contraction. In a preliminary set of experiments, in order to evaluate the nature of the phasic contractions, i.e. myogenic or neurogenic, the preparations were treated with tetrodotoxin (TTX, 1 µmol/L), a sodium channel blocking agent, commonly used to block inhibitory and excitatory motorneurons activity.
Muscle stimulation High potassium (high [K+]o, 80 mmol/L) was used as a mainly direct smooth muscle depolarizing and contracting agent without the need for receptor activation to assess whether the observed decrease in basal contractile efficacy of the circular muscle strips derived from the TNBS rats was due to a post-receptor event, i.e. intrinsic smooth muscle properties. In a set of experiments, TTX was added to the buffer in order to evaluate the potential neuro-mediated effects of high [K+]o.
Electric field stimulation (EFS) was obtained through two platin electrodes placed in parallel with respect to the circular muscle strip. The electrodes were connected to a stimulator (Mod. 1193 Tuniati, Pavia Italy) and 4 Hz stimuli were applied (25; 60 V; 0.3 ms impulses) for a total duration of 5 s. This stimulus induced a submaximal contractile response evaluated in the context of a range of stimuli applied from 1 to 15 Hz. EFS were applied every 5 min. The EFS -induced responses were neuromediated in that no effect was observed when the stimulation was performed in the presence of TTX 1 µmol/L.
Effect of L-NNA To evaluate the role of NO on basal and evoked contractile activity, the experiments were performed both in the absence and in the presence of a non-selective NOS inhibitor, L-NNA (100 µmol/L)[31]. The full effect of the compound was obtained after 10 min of incubation.
Pharmacological characterization of EFS-evoked contractile response This study was performed only in the presence of L- NNA which made responses of circular muscle strips more stable[32] and excluded the inhibitory action of NO on neuromuscular function. The major putative excitatory neurotransmitters responsible for intestinal muscle contraction after EFS are considered to be acetylcholine and tachykinins (substance P and neurokinin A)[33]. The cholinergic component of EFS contractions was determined by adding atropine (1 mol/L) to the bath. The tachykinergic component of EFS evoked contractions was established by adding neurokinin 1 (NK1) plus neurokinin 2 (NK2) receptor antagonists (0.3 µmol/L) and SR48968 (0.3 mol/L), respectively, to the bath[34,35]. SR140333 and SR48968 are selective non-peptide tachykinin NK1 and NK2 receptor antagonists, respectively[34,35]. The concentrations of these antagonists used in our study have previously been demonstrated to be effective and receptor-selective in the mammalian intestinal preparations[34,35]. The antagonists were left for at least 20 min before EFS. The antagonist concentration and time incubation were established following the demonstration of their ability to block the contractile effect of submaximal concentration of NK1 and NK2 receptor agonists, substance P methyl ester and [ALA]8-NKA[4-10] respectively, on circular muscle strips derived from controls. Atropine and NK 1 plus NK2 receptor antagonists were, sequentially, added to the bath.
The following drugs were obtained from Sigma Chemicals (Milan, Italy): atropine sulfate, TTX, L-NNA, substance P methylester and [ALA]8-NKA[4-10]. SR140333 and SR48968 were obtained by the courtesy of Dr. X. Edmond-Alt (Sanofi Research, Montpellier, France).
Substance P methylester, [ALA]8-NKA[4-10], SR140333 and SR48968 were dissolved in dimethyl sulfoxide (final solution 0.1%) and diluted in distilled water.
Statistical analysis was performed in a double-blind fashion using a suitable computer program (SAS/STAT software). Results were expressed as means ± SD. Comparison of results between groups was made using the unpaired t test, Kruskal-Wallis test, Wilcoxon rank sum test, Mann-Whitney U test, Duncan’s multiple range test, and ANOVA, when appropriate. All statistical tests were two sided. A value of P < 0.05 was considered significant.
All rats presented bloody diarrhea within 12 h of intracolonic administration of TNBS. All (n = 10) had lost 14 ± 4 g body weight, while control rats (n = 10) gained 12 ± 4 g, P < 0.01. No animal died within 48 h of colitis induction.
After laparotomy and upon visualization of the colon, TNBS rats presented a severely dilated mid-colon proximal to the grossly impaired distal colon. The ex vivo mean perimeter of the mid-colon in TNBS rats (n = 10) was 21 ± 6 mm compared to 12 ± 5 mm of the mid-colon in controls (n = 10) (P < 0.01).
In TNBS rats, a dischromic, opaque, but not perforated, serosa was observed, nearly 8 cm from the anal verge, corresponding to the contact area of the instilled TNBS solution, the lumen of the colon was entirely filled with liquid feces, while in control rats the serosa appeared normal and well-formed fecal pellets, mainly at the distal colon, were observed.
In TNBS rats, the macroscopic damage score (0 -5), at the macroscopically inflamed distal colon, was 4.1 ± 0.2 while, at the DUMC the score was 0.3 ± 0.1 (P < 0.01).
In TNBS rats, the histological evaluation showed no lesions or inflammatory infiltrate at the mid-colon, which represents the area of interest for mechanical activity studies. The distal segment was grossly ulcerated and necrotic up to the smooth muscle layers appeared to be slightly damaged. The inflammatory infiltrate was composed mainly by neutrophils and, to a lesser extent, by monocytes. The entire colon of the control rats was devoid of any significant lesions.
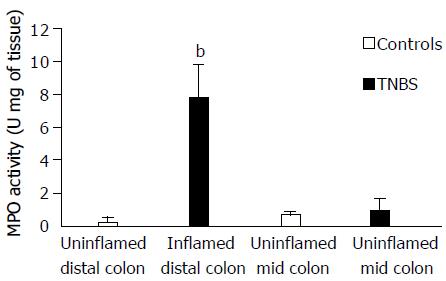
MPO activity, in the TNBS inflamed distal colon (dilated and macroscopically and histologically inflamed segment), was significantly higher than that in the distal colon of control rats (Figure 1). Whereas, in the TNBS uninflamed mid-colon (dilated but macroscopically and histological normal), MPO activity was similar to that of the corresponding mid-colon of control rats.
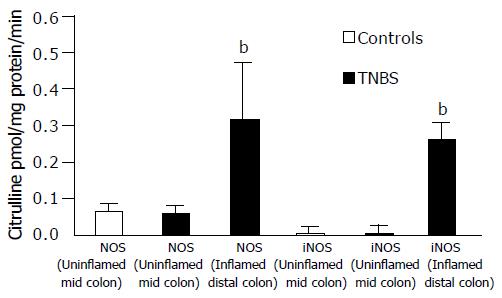
In TNBS rats, NOS activity of the inflamed distal colon was significantly increased (P < 0.01) compared to that of DUMC of TNBS and of controls (Figure 2). iNOS activity was observed only in the inflamed distal colon of TNBS rats where it was responsible for the marked increase of the overall NOS activity observed at this level. In the inflamed distal colon of TNBS rats, cNOS activity was not, in fact, significantly different from that in the controls and TNBS uninflamed mid-colon.
Spontaneous contractile activity In controls, two types of spontaneous mechanical contractions were identified, i.e., low- and high-frequency spontaneous contractions. High-frequency contractions are intermittent and produce summation contractions which are not constantly present and, therefore, these were not considered, in detail, in the present study.
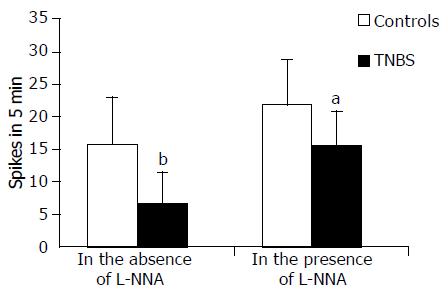
Spontaneous activity was absent in 28% of the TNBS rat DUMC preparations or they showed a significant reduction of frequency and force compared to controls (Figures 3 and 4). In both groups, the spontaneous phasic activity was significantly increased both in frequency and force by TTX (1 μmol/L), the effects being significantly greater and long lasting in the strips from the DUMC of TNBS rats than in the controls. In the former, the increase in frequency and force was 40 ± 9% and 22 ± 7%, respectively, P < 0.01; in the latter, the increase was 22 ± 8% and 14 ± 7%, respectively, P < 0.01. Furthermore, TTX evoked spontaneous contractions in 80% of specimens which, in basal conditions, appeared to be inactive. Taken together, these data highlight the greater sensitivity to neuronal inhibitory input of DUMC of TNBS vs controls.
L-NNA (100 μmol/L) induced a slow onset enhancing the effect of the frequency and force of spontaneous contractile activity which peaked after 5-7 min and remained unchanged for about 40 min in circular muscle strips from both animal groups, the effect being more pronounced in TNBS rats (Figures 3 and 4). In the presence of TTX, L-NNA induced, in both groups and to the same extent, a further slight increase (10%) in the force of muscle contraction suggesting a very small additional non-neuronal location of NO biosynthesis. L -NNA did not affect the frequency of contractions in the presence of TTX. Furthermore, L-NNA evoked phasic activity in almost all the strips (90%) from the DUMC of TNBS rats which had not originally shown any spontaneous contractions.
Overall, these findings suggest that endogenous NO negatively modulates the frequency and force of spontaneous phasic activity, and these effects were relatively more marked in tissues derived from TNBS rats than from controls.
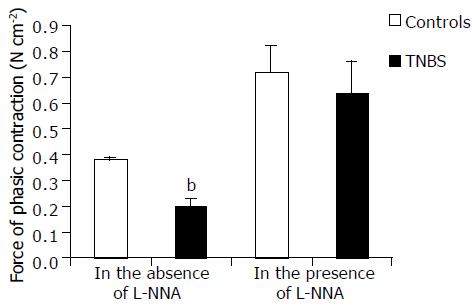
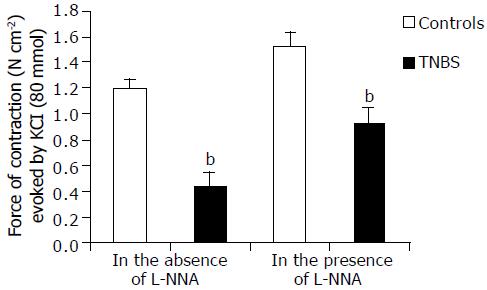
Muscle stimulation High [K+]o (80 mmol/L)-induced contractile response was quantitatively and qualitatively different in circular muscle strips from DUMC of TNBS rats compared to those from controls. In the former, the force of contraction was markedly lower (Figure 5), and its time course to peak was longer. Furthermore, in control strips, high [K+]o induced a rapid onset inhibitory response followed by contraction. In the presence of TTX, the inhibitory response was abolished indicating its neuromediated nature. The inhibitory effect to high [K+]o was seldom observed in tissues from TNBS rats probably due to their lower basal tone. In both groups, in DUMC of TNBS and controls, the contractile responses to potassium were not significantly affected by TTX, indicating that this component of potassium responses was due to a mainly direct, non-neuromediated, circular muscle activation.
In the presence of L-NNA (100 μmol/L), high [K+]o contraction was enhanced by 112 ± 22% in TNBS rats and by 28 ± 2% in control animals ( P < 0.001) (Figure 5). However, the contractile response obtained in TNBS rats remained lower [by 23.5 ± 1.8% (P < 0.01)] than in controls without L-NNA.
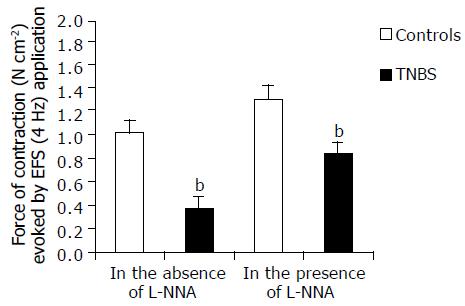
EFS (1-15 Hz) evoked frequency-dependent contractile responses both in TNBS rat colonic muscle and in that of control rats (data not shown). In this study, a submaximal frequency of stimulus of 4 Hz was used. In controls, an initial inhibitory response precedes the contraction. The force of contractions was markedly smaller in TNBS rats (Figure 6) and tended to progressively fade after further stimulations until it became stable after the third stimulus. The value of the stabilized contraction has been assumed as EFS response. EFS induced contractions, in control rats, were stable and reproducible for at least 60 min. The EFS effects were abolished by TTX thus indicating the neuromediated nature of the mechanical events (data not shown). In the presence of L-NNA, the force of contraction induced by EFS was enhanced by 121 ± 19% in TNBS rats and by 27.4 ± 1.9% in control rats (P < 0.001) (Figure 6). However, the response obtained in TNBS rats remained lower [by 17.6 ± 1.5% (P < 0.05)] than in controls without L-NNA.
In the presence of L-NNA, the inhibitory response to potassium and EFS, observed in the controls, was not significantly lesser in amplitude than in the absence of NO synthesis inhibition, indicating the relative role of NO in inducing neuromediated relaxation in these colonic preparations.
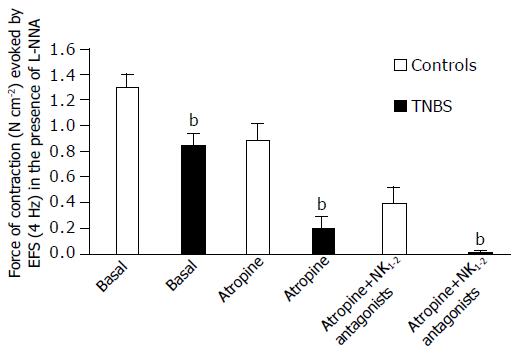
Effects of cholinergic muscarinic and NK1 plus NK2 tachykinin receptor blockade on EFS evoked circular muscle contractile response In the presence of L-NNA, in control preparations, the sequential administration of atropine (1 μmol/L) and non-peptide tachykinin NK 1 and NK2 receptor antagonists SR140333 (0.3 μmol/L) plus SR48968 (0.3 μmol/L) induced an apparent additional inhibitory effect of the EFS induced contraction leaving a non-cholinergic non-tachykinergic contractile component which was completely abolished by TTX. In TNBS rats, atropine alone induced a dramatic inhibition of contraction and the residual response was abolished by the sequential administration of SR140333 plus SR48968 (Figure 7). These results indicate the appearance of a cooperative-like interaction between the neuromuscular excitatory transmissions subserving EFS contraction in TNBS rats.
The present study focused on changes in colonic motility of the uninflamed regions of the colon observed in the course of severe human and experimental focal colitis. In rats with TNBS- induced acute distal colitis, we evaluated the spontaneous and evoked contractile activity of circular muscle strips from a region of the mid -colon, which was dilated but showed a normal appearance at light microscopy and normal MPO activity.
Low-frequency spontaneous contractile activity was always present in controls, but conversely, was absent in 28.5% of specimens from the DUMC of TNBS rats, and, if present, showed a significant reduction both in frequency and force of contraction, with respect to that in controls. Removal of the overall neural control, on smooth muscle activity, by TTX, induced an increase in the frequency and force of contractions which were significantly greater in tissues from TNBS rats and this contractile activity became present in almost all specimens. These findings confirm previous evidence that the spontaneous contractions in rat circular muscle strips, as, in general, in the mammalian colon, are myogenic in nature and under tonic neuronal inhibition[4,21]. Thus, the impairment of spontaneous activity in tissues from TNBS rats may be due to a disorder of the intrinsic muscle contractile machinery and/or due to an inappropriate neural inhibitory control.
Evoked contractions were induced by a mainly direct smooth muscle plasma membrane depolarization by high [K+]o in the buffer, and by indirect neuronal- and receptor-mediated activation of circular muscle strips by EFS, the former being largely resistant to TTX and the latter abolished by it. In the controls, both stimulatory procedures, in addition to contraction, induced a rapid onset and transient relaxation due to the release of inhibitory neurotransmitters in that it was abolished by TTX and slightly reduced by L-NNA. In the DUMC of TNBS rats, the relaxing effects were not usually observed, probably because of the lower basal tone of the circular muscle and, moreover, the high [K+]o contractions appeared markedly impaired in amplitude and slower in the time course compared to controls. Likewise, the EFS contractile responses, in TNBS rats, were smaller in force than those in controls and tended to fade at subsequent stimulations until a stable response was reached after the third stimulation.
Impairment of evoked contractions in TNBS rats may still suggest a defect of contractile machinery (as suggested by the high [K+]o response). However, a simultaneous defect in the excitatory neurotransmission (neurotransmitter release and/or receptor–postreceptor functions) in the EFS response could not be ruled out.
On the base of increasing evidence that NO plays an important role in inhibiting colonic tone and motility of the inflamed region in human and experimental animal colitis[12,17,25,26], we evaluated the potential role of NO in the impairment of muscle contractility also in the dilated uninflamed region of the TNBS rat. NO is the most important inhibitory transmitter of enteric neurons in the mammalian gut[20-22] and, in human colitis, iNANC activity has been shown to be increased[36]. Furthermore, extraneuronal NO production, by iNOS, localized in immune cells and enteric myocytes has recently been detected in inflamed colonic mucosa from patients with UC compared to controls, with significantly higher levels in TMC than in uncomplicated colitis[23-25]. Similarly, in TNBS-induced colitis, associated with colonic dilation, a striking increase in iNOS expression was found, whereas inhibition of NOS activity, by L-NNA, prevented colonic dilation[26]. The observation that interleukin 1 (IL-1), tumor necrosis factor-α (TNFα ), and interferon-γ (INFγ ), which are of prime importance in the inflammatory process of severe colitis, as well as free radicals and lipopolysaccharides, are able to induce iNOS expression in human colonic smooth muscle strips, further supports the role of iNOS in the genesis of TMC[25,26,37,38].
In this study, the role of NO on the contractility of circular muscle from DUMC of TNBS rats has been evaluated using L-NNA, a non selective NOS isoforms inhibitor. Previously, an analogous compound of L-NNA, the N-monomethyl-L-arginine, has been reported to increase spontaneous contractions of rat distal colon circular muscle strips, indicating that this colonic preparation is under tonic neural inhibition by NO biosynthesis[29]. L -NNA, added to the organ bath, induced both a relatively greater increase in the frequency and force of circular muscle phasic contractions in DUMC of TNBS than in controls and, indeed, their appearance, when absent, in the former. Furthermore, L-NNA induced a considerably greater increase of high [K+]o contractile responses in DUMC of TNBS compared to controls with a reduction in the time course. Likewise, in the presence of L-NNA, the contraction induced by EFS was enhanced in both groups, the effect being relatively more marked in strips from TNBS rats.
Studies on the pharmacological characterization of contractions induced by EFS were performed only in the presence of L-NNA in order to obtain more stable responses as already observed in rat duodenal preparations[32], and to better define potential dysfunctions in the excitatory neuro-muscular transmissions and responses, i.e., independently of the NO-mediated inhibitory action, both on excitatory neurotransmitters release and muscular contractile response. The nature of the contractile responses induced by EFS were analyzed using atropine and SR140333 plus SR48968 as selective antagonists of cholinergic muscarinic and NK1 and NK2 tachykinin receptors, respectively. In controls, a simple additional interaction was observed between the cholinergic and tachykinergic transmission in inducing contractions and the sequential blockade of the respective receptors left a non-cholinergic-non-tachykinergic contractile component which was abolished by TTX. In TNBS rats, a cooperative-like interaction between cholinergic and tachykinergic excitatory transmissions appeared to be present. The existence of a non-simple additional interaction in the latter was suggested by the evidence that the blockade of only one of the neuronal transmissions (i.e., muscarinic transmission) induced a dramatic inhibitory effect on EFS responses, and the residual contraction was completely abolished after sequential tachykinergic transmission blockade, indicating that the contribution of both the excitatory transmissions was mandatory to induce contractile responses.
The biochemical studies performed to define the activity of NOS isoforms in the DUMC tissues of TNBS showed that only cNOS was present and its activity was not different from that in control rats. In the injured distal colonic segments of TNBS rats, cNOS activity was similar to that in controls, whereas iNOS activity was markedly present, confirming previous observations[26]. These findings indicate that enhancement of spontaneous and evoked muscle contractility, in the presence of blockade of NOS activity, is due to the inhibition of the cNOS in the two groups studied. The set-up used did not allow us to characterize the cellular source and isoform of cNOS (neural or endothelial/muscular) in the colonic preparations. However, the observation that L- NNA induced a slight but not a significant increase in the amplitude of spontaneous contractions in the presence of TTX may suggest a scanty role of non-neural cNOS in the overall enzyme activity in both groups of rats. The lack of appreciable quantitative differences, in the presence and activity of cNOS, between the two groups did not allow us to give a simple interpretation for the considerably greater enhancing effect, induced by L-NNA, on the spontaneous and evoked contractions of circular muscle strips from DUMC of TNBS compared to those from controls. However, it is tempting to hypothesize that a relatively greater inhibitory effect is exerted by NO on the functionally impaired myogenic and possibly excitatory neurogenic mechanisms subserving contractions in TNBS rats. The observation that also TTX induced a considerably greater enhancing effect on spontaneous and high potassium (High[K+]o)-evoked contractions in DUMC of TNBS compared to controls may suggest that circular muscle strips from DUMC of TNBS present an abnormally high sensitivity to inhibitory neurotransmitters. On the other hand, it is unlikely that the increase in mechanical activity of the circular muscle induced by L-NNA in DUMC, is not due to the suppression of NO synthesis, but rather due to the inhibition of the production of peroxynitrite (ONOO-) in as much as the production of the latter requires the presence of the superoxide radical (.O2), (i.e. NO+.O2 →ONOO-). The generation of .O2 requires the presence of local inflammation which was absent in the intestinal preparations in which mechanical activity was evaluated.
The importance of the neurogenic mechanisms in the distension and hypocontractility of uninflamed gastrointestinal segments in TNBS- induced intestinal inflammation in rats has recently been reported[18,39].
As far as concerning the potential mechanisms involved in the impairment of intrinsic muscle contractile machinery, in the rat model of acute TNBS colitis, a decrease in the activity of smooth muscle L-type Ca++ channels has been demonstrated. Ca++ mobilization, via the L-type Ca++ channel, is the primary mechanism for excitation-contraction coupling in gastrointestinal smooth muscle. The dysfunction of the L-type Ca++ channels appears to be mediated by the activation of the NF- kB- dependent pathway leading to a marked alteration in the assessment of cytokines, as observed in acute TNBS colitis. Theoretically, the hematogenic diffusion of cytokines from the inflammatory to the non-inflammatory region of the colon may also result in the inhibition of TNBS DUMC muscle contractility as observed in the present study.
In conclusion, results emerging from the present study show that impairment of spontaneous and evoked circular muscle contraction of the DUMC in TNBS-induced distal colitis, in rats, is mainly due to a disorder of the intrinsic muscle contractile machinery. Failure of enteric neural excitatory input to the smooth muscle cannot be ruled out. Local suppression of NO synthesis markedly improves both the spontaneous and evoked muscle contractions, in spite of any evident abnormal local NO activity. New therapeutic approaches aimed at inhibition of NO synthesis or effects may improve muscle contractility, not only in the inflamed, but also in the distended uninflamed intestinal regions in the course of acute severe inflammatory bowel disease.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Fazio VW. Toxic megacolon in ulcerative colitis and Crohn's colitis. Clin Gastroenterol. 1980;9:389-407. [PubMed] |
| 2. | Sheth SG, LaMont JT. Toxic megacolon. Lancet. 1998;351:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Snape WJ, Williams R, Hyman PE. Defect in colonic smooth muscle contraction in patients with ulcerative colitis. Am J Physiol. 1991;261:G987-G991. [PubMed] |
| 4. | Koch TR, Carney JA, Go VL, Szurszewski JH. Spontaneous contractions and some electrophysiologic properties of circular muscle from normal sigmoid colon and ulcerative colitis. Gastroenterology. 1988;95:77-84. [PubMed] |
| 5. | Snape WJ, Matarazzo SA, Cohen S. Abnormal gastrocolonic response in patients with ulcerative colitis. Gut. 1980;21:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Snape WJ, Kao HW. Role of inflammatory mediators in co-lonic smooth muscle function in ulcerative colitis. Dig Dis Sci. 1988;3:65S-70S. |
| 7. | Sharkey KA. Substance P and calcitonin gene-related peptide (CGRP) in gastrointestinal inflammation. Ann N Y Acad Sci. 1992;664:425-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lennard-Jones JE, Cooper GW, Newell AC, Wilson CW, Jones FA. Observations on idiopathic proctitis. Gut. 1962;3:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Allison MC, Vallance R. Prevalence of proximal faecal stasis in active ulcerative colitis. Gut. 1991;32:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289-1297. [PubMed] |
| 11. | Caprilli R, Vernia P, Latella G, Torsoli A. Early recognition of toxic megacolon. J Clin Gastroenterol. 1987;9:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Latella G, Vernia P, Viscido A, Frieri G, Cadau G, Cocco A, Cossu A, Tomei E, Caprilli R. GI distension in severe ulcerative colitis. Am J Gastroenterol. 2002;97:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Chew CN, Nolan DJ, Jewell DP. Small bowel gas in severe ulcerative colitis. Gut. 1991;32:1535-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Caprilli R, Latella G, Vernia P, Frieri G. Multiple organ dysfunction in ulcerative colitis. Am J Gastroenterol. 2000;95:1258-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Jacobson K, McHugh K, Collins SM. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology. 1995;109:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Aube AC, Cherbut C, Barbier M, Xing JH, Roze C, Galmiche JP. Altered myoelectrical activity in noninflamed ileum of rats with colitis induced by trinitrobenzene sulphonic acid. Neurogastroenterol Motil. 1999;11:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Caprilli R, Onori L. Pathogenesis of GI distension in severe ulcer-ative colitis: a hypothesis. Gastroenterol Int. 1992;5:268-272. |
| 18. | Blandizzi C, Fornai M, Colucci R, Baschiera F, Barbara G, De Giorgio R, De Ponti F, Breschi MC, Del Tacca M. Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by alpha2-adrenoceptors in the presence of experimental colitis. Br J Pharmacol. 2003;139:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Costa M, Furness JB, Pompolo S, Brookes SJ, Bornstein JC, Bredt DS, Snyder SH. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci Lett. 1992;148:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 221] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009-1016. [PubMed] |
| 21. | Middleton SJ, Cuthbert AW, Shorthouse M, Hunter JO. Nitric oxide affects mammalian distal colonic smooth muscle by tonic neural inhibition. Br J Pharmacol. 1993;108:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Wiklund CU, Olgart C, Wiklund NP, Gustafsson LE. Modulation of cholinergic and substance P-like neurotransmission by nitric oxide in the guinea-pig ileum. Br J Pharmacol. 1993;110:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341:465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 315] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Mourelle M, Casellas F, Guarner F, Salas A, Riveros-Moreno V, Moncada S, Malagelada JR. Induction of nitric oxide synthase in colonic smooth muscle from patients with toxic megacolon. Gastroenterology. 1995;109:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Mourelle M, Vilaseca J, Guarner F, Salas A, Malagelada JR. Toxic dilatation of colon in a rat model of colitis is linked to an inducible form of nitric oxide synthase. Am J Physiol. 1996;270:G425-G430. [PubMed] |
| 27. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 28. | Wallace JL. Glucocorticoid-induced gastric mucosal damage: inhibition of leukotriene, but not prostaglandin biosynthesis. Prostaglandins. 1987;34:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Middleton SJ, Cuthbert AW, Shorthouse M, Hunter JO. Nitric oxide affects mammalian distal colonic smooth muscle by tonic neural inhibition. Br J Pharmacol. 1993;108:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Cohen JD, Kao HW, Tan ST, Lechago J, Snape WJ. Effect of acute experimental colitis on rabbit colonic smooth muscle. Am J Physiol. 1986;251:G538-G545. [PubMed] |
| 31. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] |
| 32. | Giuliani S, Tramontana M, Lecci A, Maggi CA. Tachykinin receptors mediate atropine-resistant rat duodenal reflex contractions in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Maggi CA, Zagorodnyuk V, Giuliani S. Specialization of tachykinin NK1 and NK2 receptors in producing fast and slow atropine-resistant neurotransmission to the circular muscle of the guinea-pig colon. Neuroscience. 1994;63:1137-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Emonds-Alt X, Doutremepuich JD, Heaulme M, Neliat G, Santucci V, Steinberg R, Vilain P, Bichon D, Ducoux JP, Proietto V. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur J Pharmacol. 1993;250:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 315] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Emonds-Alt X, Vilain P, Goulaouic P, Proietto V, Van Broeck D, Advenier C, Naline E, Neliat G, Le Fur G, Brelière JC. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50:PL101-PL106. [PubMed] |
| 36. | Tomita R, Munakata K, Tanjoh K. Role of non-adrenergic non-cholinergic inhibitory nerves in the colon of patients with ulcerative colitis. J Gastroenterol. 1998;33:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Cook HT, Cattell V. Role of nitric oxide in immune-mediated diseases. Clin Sci (Lond). 1996;91:375-384. [PubMed] |
| 38. | Lundberg JO, Lundberg JM, Alving K, Weitzberg E. Nitric oxide and inflammation: the answer is blowing in the wind. Nat Med. 1997;3:30-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Moreels TG, De Man JG, De Winter BY, Timmermans JP, Herman AG, Pelckmans PA. Effect of 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced ileitis on the motor function of non-inflamed rat gastric fundus. Neurogastroenterol Motil. 2001;13:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |