Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5644
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: September 28, 2005
AIM: To clarify the expression patterns and prognostic implications of the mitotic regulator Polo-like kinase 1 (PLK1) in colon cancer.
METHODS: Expression of PLK1 was investigated by immunohistochemistry (158 cases) and immunoblotting in tissue of colon adenomas and adenocarcinomas. PLK1 expression patterns were correlated with clinicopathological parameters and patient prognosis. In addition, expression of PLK1 was evaluated by immunoblot and PCR in colon carcinoma cell lines, and coexpression of PLK1 with the proliferation marker Ki-67 was investigated.
RESULTS: Weak PLK1 expression was observed in normal colon mucosa and adenomas. In contrast, 66.7% of carcinomas showed strong expression of PLK1. Overexpression of PLK1 correlated positively with Dukes stage (P < 0.001), tumor stage (P = 0.001) and nodal status (P < 0.05). Additionally, PLK1 expression was a prognostic marker in univariate survival analysis (P < 0.01) and had independent prognostic significance (RR = 3.3, P = 0.02) in patients with locoregional disease. Expression of PLK1 mRNA and protein was detected in all cell lines investigated. Coexpression of PLK1 and Ki-67 was observed in the majority of colon cancer cells, but a considerable proportion of cells showed PLK1 positivity without Ki-67 expression.
CONCLUSION: PLK1 is a new prognostic marker for colon carcinoma patients and may be involved in tumorigenesis and progression of colon cancer. Strategies focusing on PLK1 inhibition in vivo might therefore represent a promising new therapeutic approach for this tumor entity.
- Citation: Weichert W, Kristiansen G, Schmidt M, Gekeler V, Noske A, Niesporek S, Dietel M, Denkert C. Polo-like kinase 1 expression is a prognostic factor in human colon cancer. World J Gastroenterol 2005; 11(36): 5644-5650
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5644
Colorectal cancer ranks third in cancer deaths for both sexes in the Western world, with 146 940 estimated new cases and 56 730 estimated deaths in USA in 2004[1]. Incidence and mortality of colon cancer have decreased only slightly over the last 20 years. Though surgery is potentially curative, the risk of recurrence is high. Apart from surgical resection, treatment strategies for high risk patients are still mainly based on adjuvant/neoadjuvant use of 5-fluorouracil and leukovorin as chemotherapeutic agents alone or in combination with radiotherapy. Unfortunately, use of the standard therapeutic protocols results only in a moderate decline in mortality and the risk to sustain a recurrence of disease remains high[2]. Furthermore, it is still uncertain which of the patients should be treated, as prediction of the prognosis for an individual patient remains problematic[3]. With increasing knowledge about molecular changes in the malignant phenotype, therapeutic strategies focusing on targeted modulation of signal transduction pathways, which are imperative to malignant cells, are becoming increasingly important. Genetic alterations, typically associated with a malignant cell phenotype, affect genes involved in DNA repair and apoptosis, cell adhesion and invasion, angiogenesis and finally cell proliferation and cell cycle control[4].
After its discovery in Drosophila 16 years ago by Sunkel and Glover[5] polo has become the founding member of a whole family of protein kinases centrally involved in the mitotic regulation of both normal and malignant transformed cells. Since then, polo homologs have been discovered in a broad variety of species, including certain bacteria, yeast, mice, and men[6,7]. Till date, there are four known polo homologs in human beings with Polo- like kinase 1 (PLK1) being the best characterized protein of this family[8].
There is convincing evidence that PLK1 plays a central role in the G2/M transition by exerting an important control function in several steps of mitosis[7]. Additionally, PLK1 plays an important role in the regulation of microtubule dynamics and in the maturation of centrosomes[9].
Expression of PLK1 has been described in a variety of human malignancies[10-21]. We and others have reported that PLK1 overexpression had a significant impact on patient prognosis in some of these tumor entities[10,13,15,17,20] . For colon cancer, the prognostic impact of PLK1 has not been investigated so far.
The central aim of this study was to evaluate the status of PLK1 expression in a cohort of 158 benign and malignant colon tumors and in colon cancer cell lines by immunohistochemistry and immunoblotting, and to investigate the association of PLK1 expression with clinicopathological parameters and patient survival.
A total of 153 patients (age: 31-86 years, median 65.45 years) who were diagnosed for colon cancer at the Institute of Pathology, Charité University Hospital between 1996 and 1999, were included in this study. Only patients with primary colon adenocarcinomas and no other known malignancies were included. None of the patients received radiotherapy or chemotherapy prior to diagnosis. All patients were residents of the city of Berlin. The majority of patients represented consecutive cases of colon cancer in our institute. Based on tissue availability in our archive, a small number of cases (7.8%, 12 cases) had to be excluded from this study. Histologic diagnosis was established on standard H&E stained sections according to the guidelines of the World Health Organization. The details on the distribution of clinicopathological factors in the study cohort are listed in Table 1. Clinical follow-up data were available for all patients. The median follow-up time of survivors was 47 mo. Forty-one patients (27%) died after a median time of 60 mo of follow-up. As a control for non-malignant colon tumors, five adenomas of the colon were included in the study as well.
| Characteristics | All cases | PLK1negative | PLK1positive | P | |
| All cases | 153 | 51 (33.3) | 102 (66.7) | ||
| Age (yr) | |||||
| ≤65 yr | 74 (48.4) | 28 (37.8) | 46 (62.2) | 0.3041 | |
| >65 yr | 79 (51.6) | 23 (29.1) | 56(70.9) | ||
| Dukes stage | |||||
| A | 35 (22.9) | 20 (57.1) | 15 (42.9) | ||
| B | 55 (35.9) | 19 (34.5) | 36 (65.5) | <0.0012 | |
| C | 50 (32.7) | 10 (20) | 40 (80) | ||
| D | 13 (8.5) | 2 (15.4) | 11 (84.6) | ||
| Tumor stage | |||||
| T1 | 10 (6.5) | 9 (90) | 1 (10) | ||
| T2 | 36 (23.5) | 14 (38.9) | 22 (61.1) | 0.0012 | |
| T3 | 96 (62.7) | 24 (25) | 72 (75) | ||
| T4 | 11 (7.3) | 4 (36.4) | 7 (63.6) | ||
| Nodal status | |||||
| N0 | 91 (59.5) | 38 (41.8) | 53 (58.2) | ||
| N1 | 30 (19.6) | 4 (13.3) | 26 (86.7) | 0.0492 | |
| N2 | 32 (20.9) | 9 (28.1) | 23 (71.9 | ||
| State of distant metastasis | |||||
| Mx | 140 (91.5) | 49 (35) | 91 (65) | 0.2211 | |
| M1 | 13 (8.5) | 2(15.4) | 11 (84.6) | ||
| Grade | |||||
| G1 | 6 (3.9) | 2 (33.3) | 4 (66.7) | ||
| G2 | 123 (80.4) | 43 (35) | 80 (65) | 0.4232 | |
| G3 | 24 (15.7) | 6 (25) | 18 (75) | ||
The human colon carcinoma cell lines RKO and HT29 were obtained from ATCC. CX2 and HRT18 were from the German Cancer Research Center (Heidelberg, Germany). Cells were cultured in either DMEM (RKO) or RPMI (CX2, HRT18, HT29) supplemented with 10% fetal bovine serum.
Subconfluent colon carcinoma cells were harvested and total RNA was prepared with RNAeasy kit (Qiagen, Hilden, Germany) and reverse transcribed. PCR cycling conditions for PLK1 were 30 cycles of denaturation, annealing and extension at 95°C for 60 s, at 54°C for 60 s, and at 72°C for 120 s. The primers used were human PLK1 sense 5’-AGTGCTGCAGTGACTGCA- 3’ and antisense 5’-GGAGGCCTTGAGACGGTT-3’ (generating a 1 807-bp band). As control GAPDH sense 5’-ACCACAGTCC-ATGCCATCAC-3’ and antisense 5’-TCCACCACCCTG-TTGCTGTA-3’ (generating a 452-bp band) primers were used.
Tissues from colon carcinomas and normal colon mucosa were dissected by a senior pathologist in the operation theater from surgical specimens sent for frozen section analysis and were immediately frozen in liquid nitrogen and stored at -80°C until analysis.
Subconfluent colon carcinoma cells were harvested and 100 µg protein/sample was loaded on a 10% polyacrylamide gel. Western blots were performed as previously described[22], using a mouse monoclonal anti-PLK1 antibody (BD Transduction, San Diego, CA, USA) diluted 1: 500 and an anti-β-actin antibody (Chemicon, Temecula, CA, USA) diluted 1:3 000.
For immunohistochemical detection of PLK1 on tissue samples and cells, a monoclonal mouse antibody (BD Transduction) directed against human PLK1 protein was used on 5-μm paraffin sections. Antibody specificity has been ascertained by Western blot and immunohistochemistry in preceding expression studies[14,23]. For immunolabeling of Ki-67, a polyclonal rabbit antibody (Dako, Glostrup, Denmark) directed against human Ki-67 protein was used. As negative controls, slides were processed without primary antibody. For antigen retrieval, the deparaffinized slides were placed in 0.01 mol/L sodium citrate buffer, pH 6.0 and boiled for 5 min in a pressure cooker. Then slides were allowed to cool down for additional 5 min in the same buffer. After several rinses in TBS and pre-treatment with blocking reagent (Dako, Glostrup, Denmark) for 5 min, slides were incubated with primary antibody diluted 1:500 (PLK1) and 1:50 (Ki-67) in antibody diluent solution (Zymed, San Francisco, CA, USA) for 20 min at room temperature and then at 4°C overnight. After the slides were washed in TBS, bound antibody was detected by applying a streptavidin-biotin system (BioGenex, San Ramon, CA, USA) following the standard protocol with standard antibody dilutions as supplied by the manufacturers. For color development, a fast red system (Sigma, Deisenhofen, Germany) was used. The slides were mounted using Aquatex (Merck, Gernsheim, Germany).
RKO cells were grown on Labtek chamber slides. Subconfluent cells were fixed in 100% methanol for 20 min at -20°C. For immunolabeling of PLK1, slides were processed as described above but without applying the pressure cooker. After the color developed, slides were washed in PBS and subsequently incubated with a 1:50 dilution of primary antibody directed against Ki-67 for 20 min at room temperature and subsequently at 4°C overnight. After the slides were washed in TBS, bound antibody was detected by the peroxidase anti-peroxidase method following the standard protocol with standard antibody dilutions as supplied by the manufacturers (Dako). Color development was performed using a DAB+system (Dako). Finally, slides were mounted using Aquatex.
Staining intensity of tissue slides was evaluated independently by two pathologists who were blinded towards patients’ characteristics and survival. Cases with disagreement were discussed using a multi-headed microscope until agreement was achieved. To assess differences in staining intensity, an immunoreactivity scoring system (IRS) was applied. Intensity of staining was designated as negative (0), weak (1), moderate ⑵ or strong (3). Additionally, the percentage of positive cells was evaluated and scored as either no cells (0), less then 10% of cells (1), 10-50% of cells (2), 51-80% of cells ⑶ or over 80% of cells stained (4). By multiplication of these two parameters, the IRS for each case was calculated. Finally, cases were grouped as PLK negative (IRS 0-6) or PLK positive (IRS 7-12) for statistical analysis.
Statistical correlation between clinicopathological factors and expression of PLK1 was assessed by applying either Fisher’s exact test, Spearman’s rank order correlation or χ2 test for trends. The probability of differences in overall survival as a function of time was determined by Kaplan-Meier analysis and log-rank test. Multivariate probing for significance was performed applying the Cox proportional hazard model. Generally P < 0.05 was considered statistically significant. For all statistical procedures, SPSS v10.0 software was used.
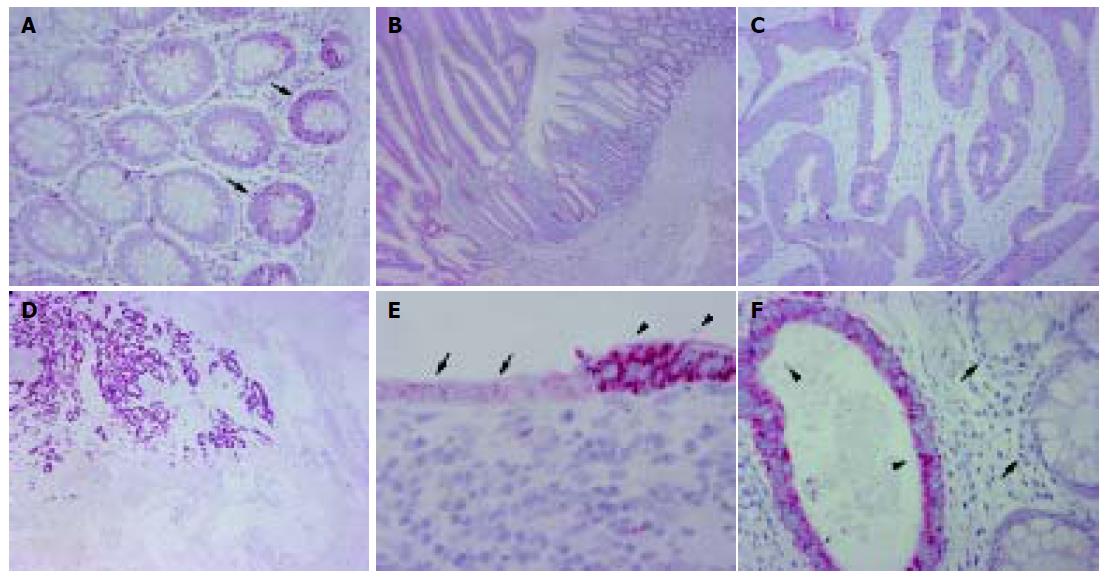
Normal colon mucosa from both the vicinity of benign and malignant tumors as well as from more distant sites showed a weak cytoplasmic staining of the epithelium at the basis of colon crypts (Figure 1). Staining was lost in the epithelium of apical parts of the crypts. A comparable staining in the epithelium was observed on serial sections for the proliferation marker Ki-67 (data not shown).
Occasionally, lymphocytes resident in normal colon mucosa showed moderate staining for PLK1 in the cytoplasm. Moderate PLK1 positivity was observed in autonomous neural plexus cells in the submucosa and muscularis propria of the colon. This staining served as an internal positive control.
None of the five adenomas of colon mucosa showed sufficient staining of PLK1 to be designated as PLK1 positive, although a weak patchy staining in the epithelium was observed in all cases (Figure 1).
Among the 153 colon cancers investigated, 102 (66.7%) were PLK1 positive in the neoplastic epithelium (Figure 1 and Table 1). Staining was inhomogeneous in a considerable number of cases with pronounced PLK1 positivity at the leading edge of tumor invasion. However, cases scored as PLK1 negative consistently showed a weak irregular staining for PLK1 in the cytoplasm as well (Figure 1). The tumor-associated inflammatory infiltrate occasionally expressed moderate amounts of PLK1, while all other stromal cells were PLK1 negative in all cases.
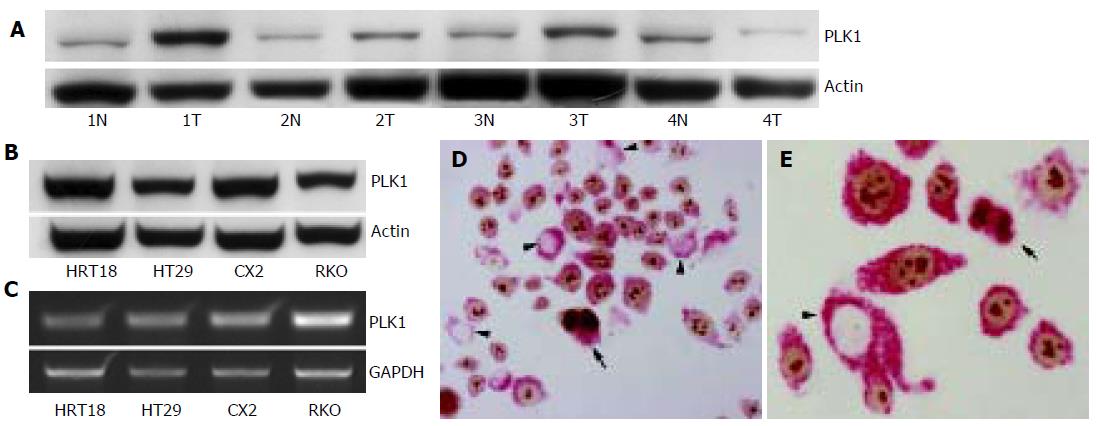
Overexpression of PLK1 in colon carcinoma compared to normal colon mucosa could be confirmed by immunoblotting in three of four matched samples of normal colon mucosa and colon carcinoma (Figure 2). In one case the tumor tissue showed a loss of expression in comparison to the normal colon mucosa.
In addition to the investigation of primary colon carcinoma tissue, we also tested four colon carcinoma cell lines for expression of PLK1 mRNA and protein. As shown in Figure 2, all cell lines expressed PLK1 mRNA measured by RT-PCR with a 1 807-bp band. On the protein level, PLK1 expression with an estimated size of 68 ku was detected in all cell lines by immunoblot.
Since PLK1 plays a pivotal role in cell cycle control, we investigated the coexpression of Ki-67 and PLK1 in the colon cancer cell line RKO by immunolabeling both proteins consecutively on the same slide. As illustrated in Figure 2, simultaneous expression of PLK1 in cytoplasm and Ki-67 in nuclei was evident in the majority of cells. However, while almost all cells expressed PLK1, there was a considerable fraction of cells without simultaneous Ki-67 expression. Similar results were observed in serial sections of primary colon carcinoma tissue. Staining of serial sections of colon carcinomas with PLK1 and Ki-67 revealed no strict overlap between the expressions of both proteins though highly proliferating tumors (strong Ki-67 positivity) were more prone to overexpress PLK1.
A highly significant positive correlation of PLK1 expression with either Dukes stage (P < 0.001, χ2 test for trends) and WHO tumor stage (P = 0.001, χ2 test for trends) could be observed. More advanced tumors expressed significantly higher amounts of PLK1 than locally restricted carcinomas (Table 1). Additionally, a significant positive correlation of PLK1 expression with nodal status could be demonstrated (P = 0.049, χ2 test for trends). This observation was further confirmed by a significant positive correlation (P = 0.044, r = 0.163, Spearman’s rank order correlation) of the number of positive lymph nodes with ungrouped PLK1 score. We did not observe a correlation of PLK1 expression in colon cancer with patients’ age, status of distant metastasis and tumor grade (Table 1)
Known prognostic parameters in colon cancer, as patients’ age, Dukes stage, WHO tumor stage, lymph node status, status of distant metastasis and tumor grade were confirmed to have a significant impact on patient prognosis in this study (Table 2).
| Cases | Events | Mean survival(mo) | Standard error | Log-ranktest P | ||
| Age (yr) | ≤65 yr | 74 | 13 | 76.59 | 3.42 | 0.0142 |
| >65 yr | 79 | 29 | 57.94 | 3.51 | ||
| Dukes stage | A | 35 | 4 | 70.27 | 3.18 | <0.0001 |
| B | 55 | 5 | 83.89 | 2.67 | ||
| C | 50 | 23 | 48.26 | 4.28 | ||
| D | 13 | 10 | 22.96 | 6.6 | ||
| Tumor stage | T1 | 10 | 0 | Not reached | – | 0.0006 |
| T2 | 36 | 8 | 74.08 | 5.06 | ||
| T3 | 96 | 27 | 63.84 | 3.06 | ||
| T4 | 11 | 7 | 27 | 7.22 | ||
| Nodal status | N0 | 91 | 11 | 81.93 | 2.34 | <0.0001 |
| N1 | 30 | 10 | 55.94 | 5.17 | ||
| N2 | 32 | 21 | 28.74 | 3.97 | ||
| Metastasis | Mx | 140 | 32 | 73.19 | 2.68 | <0.0001 |
| M1 | 13 | 10 | 22.96 | 6.6 | ||
| Grade | G1 | 6 | 0 | Not reached | – | 0.0007 |
| G2 | 123 | 29 | 72.61 | 2.9 | ||
| G3 | 24 | 13 | 33.37 | 4.68 | ||
| PLK1 | Negative | 51 | 7 | 74.13 | 2.9 | 0.006 |
| (all cases) | Positive | 102 | 35 | 63.75 | 3.66 | |
| PLK1 | Negative | 49 | 5 | 76.36 | 2.52 | |
| (locoregional disease) | Positive | 91 | 27 | 67.77 | 3.66 | 0.0119 |
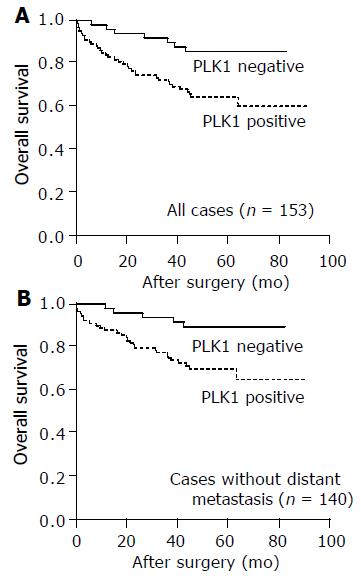
Additionally, PLK1 expression had a significant impact on patient prognosis both in the whole study cohort (n = 153, P = 0.006) and in the subgroup of patients without distant metastasis at the time of diagnosis (n = 140, P = 0.012). The mean survival time of patients in the PLK1 negative groups was 74.13 mo (whole group) and 76.36 mo (subgroup without distant metastasis), while the survival time was reduced to a mean of 63.75 mo (whole group) and 67.77 mo (subgroup without distant metastasis) in the PLK1 positive groups, respectively (Figure 3). In the whole study cohort, patients with PLK1 positive tumors showed a rate of 65% survival after 5 years compared to 86% survival in the PLK1 negative group. In the subgroup without distant metastasis, the 5-year survival was reduced from 89% in the PLK1 negative group to 70% in the PLK1 positive group (Table 2).
In the whole study population, PLK1 expression failed to be a significant independent predictor of prognosis (P = 0.091, RR = 2.1). Independent prognostic predictors were patients’ age, nodal status, state of distant metastasis and tumor grade (Table 3). In contrast, in the subgroup of patients without distant metastasis, PLK1 expression had a significant impact on patient prognosis in multivariate survival analysis. Patients with PLK1 positive tumors were approximately thrice (RR = 3.3, P = 0.019) more likely to die in the given time interval compared to their PLK1 negative counterparts. Again, patients’ age, nodal status and tumor grade remained independent significant predictors of prognosis (Table 4).
| RR | 95%CI | P | |
| Age (yr) | |||
| per yr | 1.040 | 1.008–1.072 | 0.013 |
| Tumor stage | |||
| T1/T2 | 1.000 | ||
| T3/T4 | 1.061 | 0.466–2.413 | 0.888 |
| Nodal status | |||
| Per positive node | 1.116 | 1.037–1.201 | 0.003 |
| Metastasis | |||
| Mx | 1.000 | ||
| M1 | 3.223 | 1.202–8.643 | 0.02 |
| Grade | |||
| G1/G2 | 1.000 | ||
| G3 | 2.773 | 1.382–5.564 | 0.004 |
| PLK1 expression | |||
| Negative | 1.000 | ||
| Positive | 2.067 | 0.890–4.800 | 0.091 |
| RR | 95%CI | P | |
| Age (yr) | |||
| per yr | 1.044 | 1.009–1.079 | 0.013 |
| Tumor stage | |||
| T1/T2 | 1.000 | ||
| T3/T4 | 0.898 | 0.365–2.206 | 0.814 |
| Nodal status | |||
| Per positive node | 1.231 | 1.125–1.348 | <0.001 |
| Grade | |||
| G1/G2 | 1.000 | ||
| G3 | 2.614 | 1.172–5.829 | 0.019 |
| PLK1 expression | |||
| Negative | 1.000 | ||
| Positive | 3.306 | 1.214–9.002 | 0.019 |
To our knowledge, this is the first study showing an adverse effect of PLK1 overexpression on the prognosis of patients with colon cancer. This effect remains independently significant in the clinically important subgroup of patients without known distant metastasis.
In the present study, PLK1 was overexpressed in 66.7% of colon cancers and overexpression was positively linked to tumor stage and lymph node status, two parameters
that determine the extent of tumor burden. This is in line with the results of Takahashi et al[21]. In another study by Macmillan et al[24], increased PLK1 transcription levels were observed in colon carcinoma.
PLK1 overexpression in malignancies seems to be a common event, since it has been observed in a variety of different neoplasms, including cancer of stomach[10], esophagus[10], breast[11], endometrium[12], ovary[13], brain[14], head and neck[15], skin[16], lung[17], prostate[18], thyroid[19], liver[20], and colon[21]. Effects of PLK expression on patient prognosis have already been reported in a variety of other tumor entities, including esophageal carcinoma[10], ovarian carcinoma[13], carcinoma of head and neck[15], non-small cell lung carcinomas[17], and hepatoblastoma[20].
Among the possible functions of PLK1 overexpression in tumor biology, the most important factor might be the central role of PLK1 in mitotic regulation. PLK1 initiates mitosis by the activation of cdc25 and cyclin B1 via direct phosphorylation. Both events lead to an accumulation of cdc2/cyclin B1 complex in the nuclei, a precondition known to be crucial for the onset of mitosis[25,26]. Later in mitosis, PLK1 triggers the dissociation of cohesins from their chromosomal binding sites[27] and activates the anaphase-promoting complex[28]. The central involvement of PLK1 in mitosis of malignant cells is supported bys findings in several tumor entities including colon cancer[21], showing that PLK1 expression correlated positively with markers of cell proliferation.
Nevertheless, there are several arguments supporting the theory that additional functions of PLK1 must exist. First, only a part of the variety of PLK1 expression can be explained by mitotic activity. Second, we observed expression of PLK1 in irreversibly postmitotic cells such as neuronal ganglion cells. Third, PLK1 expression is unlikely to reflect cell proliferation exclusively, since we observed cases with strong PLK1 expression in almost 100% of tumor cells (Figure 1), a number that by far exceeds the rate of cells that can be expected to be in G2/M phase. Finally, we could show that PLK1 expression is not strictly confined to cycling cells by double staining of colon cancer cells. Additional functions of PLK1 in tumor biology may have something to do with its ability to control microtubule dynamics[9], which is not only essential in mitosis but also important in golgi protein transport and cytoskeletal formation and rearrangement. These possible additional roles of PLK1 overexpression in tumor biology should be subjected to further functional studies.
Another explanation for the cause of PLK1 deregulation might be that either enhanced transcription by altered intracellular signal transduction or chromosomal over-representation of the respective gene locus leads to high cellular protein levels, which in turn enhance mitotic activity. In fact, chromosomal amplifications of 16p12, the gene locus of PLK1 has been described in human colorectal carcinomas[29].
Most recently, several groups found that inhibition of PLK1 expression in cancer cells of various tumor entities leads to a dramatic decline in cell proliferation[23,30] and to an induction of apoptosis[31]. These results suggest that PLK1 is an attractive target for novel chemotherapeutic approaches.
Expression studies, like ours, may thus provide the translational basis for such new therapeutical approaches.
In summary, PLK1 is overexpressed in 66.7% of colon cancers, overexpression of PLK1 is linked to advanced tumor stages in this disease and can be considered as a new independent prognostic marker for colon cancer patients with locoregional disease.
The authors thank Lisa Glanz for excellent technical assistance and Martina Eickmann for help in the preparation of the manuscript.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3094] [Cited by in RCA: 2973] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 2. | Chau I, Chan S, Cunningham D. Overview of preoperative and postoperative therapy for colorectal cancer: the European and United States perspectives. Clin Colorectal Cancer. 2003;3:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Cascinu S, Georgoulias V, Kerr D, Maughan T, Labianca R, Ychou M. Colorectal cancer in the adjuvant setting: perspectives on treatment and the role of prognostic factors. Ann Oncol. 2003;14 Suppl 2:ii25-ii29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19494] [Article Influence: 779.8] [Reference Citation Analysis (0)] |
| 5. | Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89:25-38. [PubMed] |
| 6. | Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 265] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 887] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 8. | Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5931] [Cited by in RCA: 5961] [Article Influence: 259.2] [Reference Citation Analysis (0)] |
| 9. | Dai W, Wang Q, Traganos F. Polo-like kinases and centrosome regulation. Oncogene. 2002;21:6195-6200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999;15:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, Strebhardt K, Bleyl U. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract. 2000;196:753-759. [PubMed] |
| 12. | Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Polo-like kinase (PLK) expression in endometrial carcinoma. Cancer Lett. 2001;169:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Köbel M, Dietel M, Hauptmann S. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer. 2004;90:815-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Dietzmann K, Kirches E, von Bossanyi K, Mawrin C. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001;53:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794-2797. [PubMed] |
| 16. | Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol. 2002;29:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, Altmannsberger HM, Rübsamen-Waigmann H, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 278] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Weichert W, Schmidt M, Gekeler V, Denkert C, Stephan C, Jung K, Loening S, Dietel M, Kristiansen G. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate. 2004;60:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Ito Y, Miyoshi E, Sasaki N, Kakudo K, Yoshida H, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K. Polo-like kinase 1 overexpression is an early event in the progression of papillary carcinoma. Br J Cancer. 2004;90:414-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Yamada S, Ohira M, Horie H, Ando K, Takayasu H, Suzuki Y, Sugano S, Hirata T, Goto T, Matsunaga T. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: identification of high expression of the PLK1 oncogene as a poor-prognostic indicator of hepatoblastomas. Oncogene. 2004;23:5901-5911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Takahashi T, Sano B, Nagata T, Kato H, Sugiyama Y, Kunieda K, Kimura M, Okano Y, Saji S. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci. 2003;94:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Denkert C, Köbel M, Pest S, Koch I, Berger S, Schwabe M, Siegert A, Reles A, Klosterhalfen B, Hauptmann S. Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma. Am J Pathol. 2002;160:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Elez R, Piiper A, Kronenberger B, Kock M, Brendel M, Hermann E, Pliquett U, Neumann E, Zeuzem S. Tumor regression by combination antisense therapy against Plk1 and Bcl-2. Oncogene. 2003;22:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol. 2001;8:729-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 248] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Yuan J, Eckerdt F, Bereiter-Hahn J, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene. 2002;21:8282-8292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Golan A, Yudkovsky Y, Hershko A. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J Biol Chem. 2002;277:15552-15557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Knösel T, Schlüns K, Stein U, Schwabe H, Schlag PM, Dietel M, Petersen I. Chromosomal alterations during lymphatic and liver metastasis formation of colorectal cancer. Neoplasia. 2004;6:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Spänkuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst. 2002;94:1863-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 261] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA. 2003;100:5789-5794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |