Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5607
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: September 28, 2005
AIM: To investigate the usefulness of 1.5 Harmonic Imaging Sonography with the use of the contrast agent Levovist for the diagnosis of hepatocellular carcinoma (HCC) and for the evaluation of therapeutic response.
METHODS: Phantom experiments were performed to compare the contrast effects of 2nd harmonic imaging and 1.5 Harmonic Imaging Sonography. 1.5 Harmonic Imaging Sonography was employed to examine 36 patients with HCC (42 nodules) before and after the treatment and to compare against the findings obtained using other diagnostic imaging modalities.
RESULTS: In 1.5 Harmonic Imaging Sonography, the tumor vessels of HCCs were clearly identified during the early phase, and late-phase images clearly demonstrated the differences in contrast enhancement between the tumor and surrounding hepatic parenchyma. Blood flow within the tumor was detected in 36 nodules (85.7%) during the early phase and in all 42 nodules (100%) during the late phase using 1.5 Harmonic Imaging Sonography, in 38 nodules (90.5%) using contrast-enhanced CT, in 34 nodules (81.0%) using digital subtraction angiography (DSA), and in 42 nodules (100%) using US CO2 angiography. Following transcatheter arterial embolization, 1.5 Harmonic Imaging Sonography detected blood flow and contrast enhancement within the tumors that were judged to contain viable tissue in 20 of 42 nodules (47.6%). However, 6 of these 20 cases were not judged in contrast-enhanced CT. 1.5 Harmonic Imaging Sonography was compared with the US CO2 angiography findings as the gold standard, and the sensitivity and specificity of these images for discerning viable and nonviable HCC after transcatheter arterial embolization were 100% and 100%, respectively.
CONCLUSION: 1.5 Harmonic Imaging Sonography permits the vascular structures of HCCs to be identified and blood flow within the tumor to be clearly demonstrated. Furthermore, 1.5 Harmonic Imaging Sonography is potentially useful for evaluating the therapeutic effects of transcatheter arterial embolization on HCC.
- Citation: Yamamoto K, Shiraki K, Nakanishi S, Fuke H, Nakano T, Hashimoto A, Shimizu A, Hamataki T. 1.5 Harmonic Imaging Sonography with microbubble contrast agent improves characterization of hepatocellular carcinoma. World J Gastroenterol 2005; 11(36): 5607-5613
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5607
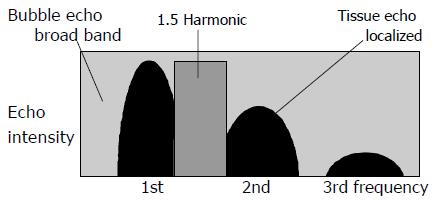
The usefulness of the peripheral ultrasound contrast agent Levovist was originally thought to be limited to signal enhancement in color/power Doppler ultrasonography[1,2]. Levovist is now employed for the diagnosis of hepatic tumors, in which it has been found to enhance blood flow signals and thus improve the visualization of hepatic tumor vessels[3-5]. However, recent advances in ultrasonographic technologies have led to the development of new imaging techniques collectively referred to as “harmonic imaging”, in which images are obtained using the 2nd harmonic components of the ultrasound signal. Among these new techniques, flash echo imaging (FEI) employs intermittent transmission at high acoustic power to permit the microbubbles to enter the scanning plane and thus to obtain echoes with high sensitivity. In other words, FEI makes it possible to acquire detailed images with high spatial resolution in which only blood flow information is extracted, making it easier to visualize tumor vessels. In contrast ultrasonographic examination of hepatocellular carcinoma (HCC), the microbubbles in Levovist cause higher frequency signals to be generated by the tumor than by normal hepatic parenchyma. Consequently, the contrast resolution of the tumor and normal parenchyma is further improved and minute blood vessels within the tumor can be visualized[6-11]. One technique is digital subtraction imaging, which permits the contrast effect to be clearly identified and is therefore employed for the diagnosis of hepatic tumors[12-15]. In 2nd harmonic imaging, however, the presence of tissue harmonic components may lead to poorer contrast enhancement in some cases. In another method, Doppler technology is used to eliminate tissue echoes and to visualize the nonlinear behavior of the microbubbles[16-18], but this method is susceptible to motion artifacts near the heart. A new technology known as 1.5 Harmonic Imaging Sonography has been developed to overcome these problems. In this method, images are obtained using a band that is intermediate between the fundamental and the 2nd harmonic components (Figure 1). In the 1.5 Harmonic Imaging technique, a frequency band whose center frequency is higher than the fundamental by a factor of 3/2 is extracted and visualized. This frequency band is intermediate between the fundamental and the second harmonic. It includes only bubble echoes and is free of tissue echoes. This imaging technique reduces tissue echoes without generating motion artifacts such as those that are seen in the pseudo-Doppler imaging technique. Contrast between tissues and bubbles is improved by 20 dB or more compared with the 2nd harmonic imaging technique. 1.5 Harmonic Imaging Sonography involves the use of a transmission waveform in which the bandwidth is limited and the leakage of fundamental components is therefore reduced. The fundamental components of tissue echoes are effectively separated from the 2nd harmonic components, permitting images to be obtained in an intermediate band that is almost completely free from tissue echoes. When images are obtained in this intermediate band, higher contrast can be achieved between contrast agent and tissue echoes than in conventional 2nd harmonic imaging. The echo from the tissue has only fundamental and 2nd harmonic component. In the intermediate band, there exists no tissue echo with the dedicated transmission where the fundamental band width and leakage are well controlled. On the other hand, bubble echo has broadband harmonics in high mechanical index (MI) contrast imaging. So high bubble/tissue signal ratio is achieved in image with the component of that intermediate band. Nevertheless, to our knowledge, there have been no studies on the clinical application of 1.5 Harmonic Imaging Sonography for the evaluation of hepatic tumors and the assessment of therapeutic effects. The present study was therefore conducted to investigate the clinical usefulness of the ultrasound contrast agent Levovist for the examination of HCCs and for the assessment of therapeutic effects.
This study was performed with approval from the institutional review board, and informed consent was obtained from each patient before undergoing this procedure.
From September 2002 and November 2003, we enrolled in this study 36 patients with unresectable HCC, who had a total of 42 hypervascular HCC nodules. The subjects in the present study were 36 patients with 42 nodules that were diagnosed as HCC based on histopathological examination (n = 18) and diagnostic imaging findings (n = 24). The study group comprised 36 patients with type C cirrhosis: 24 men and 12 women ranging in age from 41 to 78 years and with a mean age of 62 years. The minimum tumor diameter was 10 mm, the maximum tumor diameter was 73 mm, and the mean tumor diameter was 25.8±15.1 mm (±SD). All those HCC nodules have been diagnosed by the sonography. Before therapy, serum α-fetoprotein levels (normal, <7 ng/mL) ranged from 4.7 to 928.6 ng/mL (mean, 186.1 ng/mL). 12/36(33.3%) case showed normal α-fetoprotein level.
Contrast-enhanced sonography examination Ultrasound images were acquired using a diagnostic ultrasound system (SSA-770A, Aplio, Toshiba) with phased-array transducers operating at frequencies of approximately 2.5 and 3.0 MHz (PST-25AT and PST-30AT sector transducers, Toshiba). The MI was set to 1.6, and the transmission/reception frequencies were 1.4 MHz/3.0 MHz for 2nd harmonic imaging and 2.3 MHz/3.3 MHz in phantom experiments and 1.7 MHz/2.5 MHz in clinical study for 1.5 Harmonic Imaging Sonography. Contrast images were acquired using FEI.
Phantom experiments were conducted to compare images obtained by 2nd harmonic imaging and 1.5 Harmonic Imaging Sonography (i.e., to compare tissue and microbubble echo contrast between 2nd harmonic imaging and 1.5 Harmonic Imaging Sonography). The phantom consisted of agar mixed with powdered graphite to simulate attenuation in the living body (Nihonkai Medical; 0.6, 0.9 dB/cm/MHz). The tube in this phantom was filled with the ultrasound contrast agent Levovist, which contains particles composed of a mixture of galactose and palmitic acid (SHU 508A, Levovist, Schering, Berlin, Germany; concentration 300 mg/mL). Images of this phantom were acquired using FEI.
In the clinical study, a total of 7 mL of Levovist at a concentration of 300 mg/mL was injected as a single bolus via a peripheral vein at a rate of 1 mL/s. Images were acquired using FEI at intervals of 0.2 s. The early phase was specified as the period of up to 60 s after the injection of contrast agent, and the tumor vessels were observed with the focal point set at the bottom of the tumor. Then, after an interval of 4 min, the late phase was specified as the period beginning at the 5-min time point, and ultrasound transmission was performed once manually to observe vascular enhancement (staining) of the tumor tissues. Images were recorded on S-VHS videotape immediately after the injection of contrast agent. The patient was instructed to hold his or her breath for 10-50 s after the injection of Levovist in order to maintain a constant respiratory phase, and early-phase images were obtained continuously. Subsequently, the system was temporarily set to freeze mode and the images stored in cine loop memory were recorded onto the system hard disk. Five minutes after the injection, late-phase images were observed for several seconds during breath-holding and recorded onto the system hard disk.
Contrast-enhanced CT Contrast-enhanced CT was performed in all patients before treatment. Contiguous 5-mm-thick axial CT scans were obtained with a HiSpeed Advantage RP scanner (Lemage Supreme, General Electric Yokogawa Medical Systems, Tokyo, Japan). A nonionic contrast material, 100 mL of iopamidol (Iopamiron, Nippon Schering, Osaka, Japan), was administered at a rate of 3 mL/s, and CT scans of the entire liver were obtained twice: first at 20-40 s (arterial phase) after IV injection of contrast material and then at 90 s (portal phase). Lesions were considered to be hypervascular if they appeared denser than the surrounding liver during the arterial phase.
Digital subtraction angiography Digital subtraction angiography (DSA) images were obtained using a DSA system (Infinix NB, Toshiba). The contrast agent was injected via a catheter placed in the hepatic artery. The presence or absence of tumor vessels was assessed. When tumor vessels were identified, the tumor was considered to be hypervascular. We performed transcatheter arterial chemoembolization in all patients by selectively introducing a coaxial microcatheter into a segmental branch or a subsegmental branch of the hepatic artery and injecting a mixture of iodized oil (Lipiodol, Guerbet, Aulnay-sous-Bois, France; 3.0-10.0 mL/body) or epirubicin hydrochloride (Farmorubicin, Pharmacia and Upjohn, Tokyo, Japan: 30- 60 mg per body weight). We then prefor med embolization using 0.5 mm×0.5 mm×0.5 mm gelatin sponge (Spongel, Yamamouchi Pharmaceuticals) in all patients. These embolic materials were injected until the feeding arteries were completely obliterated. If the blood supply was from two different subsegmental arteries, both were embolized. The effectiveness of transcatheter arterial chemoembolization was evaluated by 1.5 Harmonic Imaging Sonography and contrast-enhanced CT at 7 d after treatment. US CO2 angiography In ultrasound angiography, after the tumor was identified in a cross-sectional ultrasound image, CO2 microbubbles were injected at a constant rate during breath-holding via a catheter placed in the hepatic artery. The CO2 microbubbles were prepared by adding 5 mL of autologous blood to 10 mL of CO2, connecting the container to a three-way stopcock, and transferring the mixture repeatedly approximately 30 times with a pump. Ultrasound images were acquired using a diagnostic ultrasound system (SSA-250A, Toshiba) with a 3.75-MHz convex transducer. Images were simultaneously recorded on videotape and magneto-optical disk from the time immediately before the injection of CO2 microbubbles until the time when tumor enhancement was observed. When the tumor showed stronger enhancement than the surrounding hepatic parenchyma, it was considered to be hypervascular.
CT during arterial portography CT during arterial portography (CTAP) was performed using a CT scanner (Asteion, Toshiba, Tokyo Japan). For CTAP, 100 mL of Iopamidol contrast medium (Iopamiron 150; Nihon Schering, Osaka, Japan) was administered via a catheter in the superior mesenteric artery at an estimated rate of 2.0 mL/s during sequential helical scanning of the liver. For CT arteriography, 30-50 mL of contract medium (Iopamiron 150) was administered via a catheter in the proper hepatic, left, or right hepatic artery at an estimated rate of 1.5-2.5 mL/s during sequential helical scanning of the liver. Tumors that did not contain portal blood flow were identified as low-absorption areas in the hepatic parenchyma (portal perfusion defects).
Imaging analysis All the sonographic data were recorded on videotape from the beginning of B-mode scanning. The still images were stored on the hard disk of the unit by reviewing the cine loop memory. The images obtained using the above modalities were interpreted by three specialists in diagnostic imaging. When a discrepancy occurred among the interpreters, re- evaluation and discussion were done for agreement. On contrast-enhanced imaging with 1.5 Harmonic Imaging Sonography, the presence of the intratumoral blood flow signals on either the vessel image or on the tumor parenchymal stain image was considered as hypervascular or residual tumor due to inadequate treatment. In contrast, the absence of the intratumoral blood flow signals was evaluated as hypovascular or complete response to the treatment. The contrast enhancement on CT was reviewed by three radiologists without knowledge of the 1.5 Harmonic Imaging Sonography results.
The sensitivity and specificity of 1.5 Harmonic Imaging Sonography in detecting intratumoral blood flow signals on the vessel tumor were determined using US CO2 angiography as a gold standard[21,22]. The detectability of residual tumor using contrast-enhanced 1.5 Harmonic Imaging Sonography was compared with that of the tumors using contrast-enhanced CT.
The data were analyzed with the χ2 test. A P value less than 0.05 was considered significant.
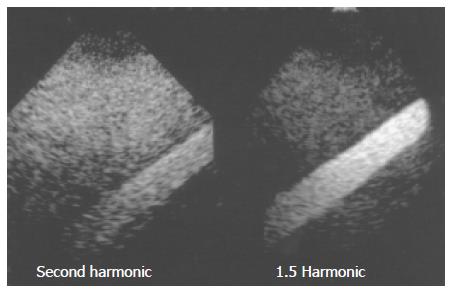
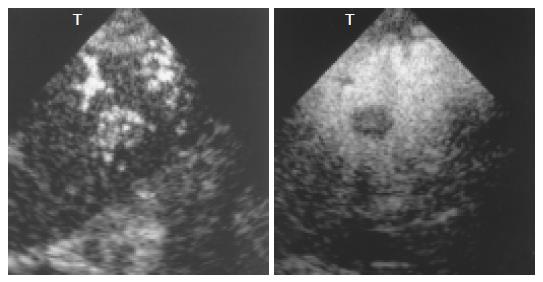
In the phantom experiments, the area occupied by the contrast agent was shown as a gleaming white zone, with the surrounding area corresponding to a tissue-equivalent phantom composed of agar mixed with powdered graphite. In oblique images, the echoes from the microbubbles appeared as an oblique bright zone, and the surrounding dark area corresponded to echoes from the agar phantom. The images clearly demonstrated the improvement in tissue-microbubble contrast in 1.5 Harmonic Imaging Sonography as compared with 2nd harmonic imaging. In 1.5 Harmonic Imaging Sonography, the contrast between tissues and microbubbles was found to be clearly superior to that in 2nd harmonic imaging (Figure 2).
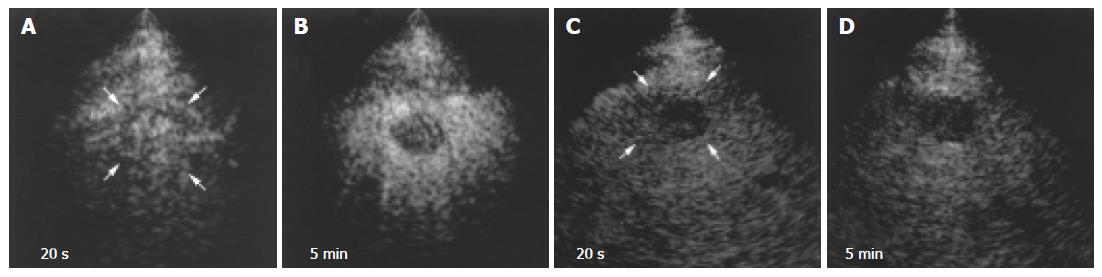
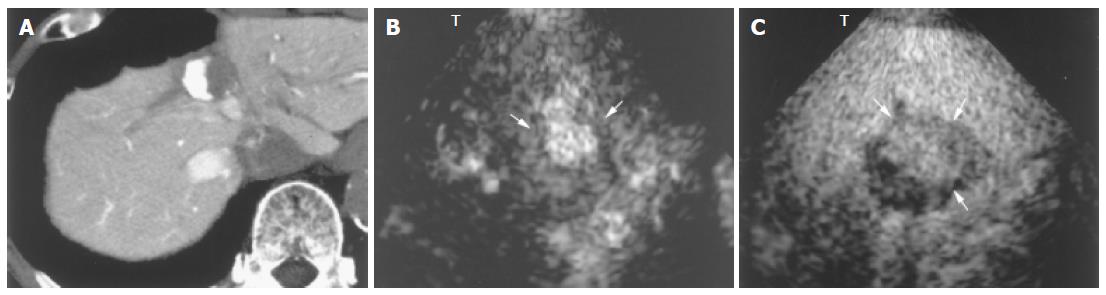
Blood vessels in all HCCs were more clearly depicted by 1.5 Harmonic Imaging Sonography than by 2nd harmonic imaging (Figures 3A and C). Moreover, during the late phase, 1.5 Harmonic Imaging Sonography demonstrated the surrounding hepatic parenchyma with higher contrast enhancement than 2nd harmonic imaging, clearly depicting the area of the tumor (Figures 3B and D).
In hypervascular HCCs, the blood vessels within the tumor were clearly demonstrated during the early phase (Figures 4B-D and 5A). In addition, late-phase images revealed partial residual enhancement in all tumors, but with less enhancement than the surrounding hepatic parenchyma (Figures 3B and 5B).
With regard to the detection of blood flow signals within the tumor in the 42 nodules, early- phase 1.5 Harmonic Imaging Sonography revealed the tumor vessels in 36 nodules (85.7%). Late-phase images demonstrated significantly lower enhancement of the tumor than the surrounding hepatic parenchyma in all 42 nodules (100%). Early -phase 1.5 Harmonic Imaging Sonography did not show hypervascular enhancement in six nodules (14.3%), and late-phase images confirmed that these six nodules were not enhanced (P > 0.05). As for the findings of other imaging modalities, blood flow signals were detected within the tumor in 39 nodules (92.9%) by contrast-enhanced CT, in 34 nodules (81.0%) by DSA, and in 42 nodules (100%) by US CO2 angiography. CTAP showed a perfusion defect image in all 42 nodules (100%, Table 1).
| 1.5 Harmonic (early phase)enhancement | Imaging sonography (late phase)no enhancement | CTAP | US CO2angiography | DSA | CECT | |
| Positive cases | 36 | 42 | 42 | 42 | 34 | 39 |
| (%) | (85.7) | (100) | (100) | (100) | (81.0) | (92.9) |
Contrast-enhanced CT did not show hypervascular enhancement in three nodules (one small HCC nodule measuring 10 mm in diameter and two nodule near the heart), but 1.5 Harmonic Imaging Sonography was able to demonstrate the tumor vessels even in these three nodules. Furthermore, DSA failed to demonstrate tumor vessels in eight nodules, while these vessels were successfully visualized by 1.5 Harmonic Imaging Sonography. 1.5 Harmonic Imaging Sonography were compared with the US CO2 angiography findings as the gold standard, and the sensitivity and specificity of these images for discerning viable and nonviable HCC after transcatheter arterial embolization were 100% and 100%, respectively.
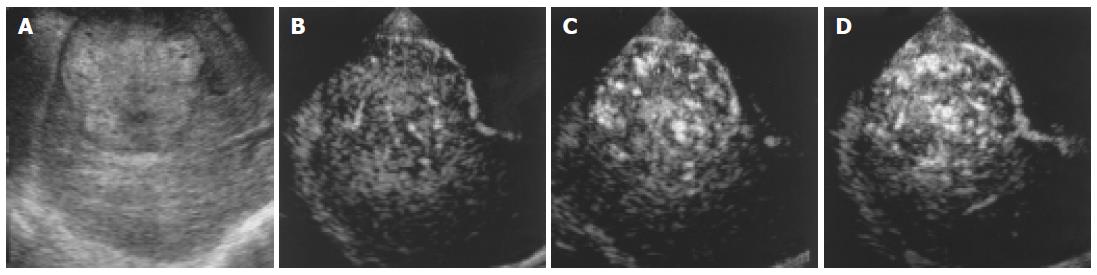
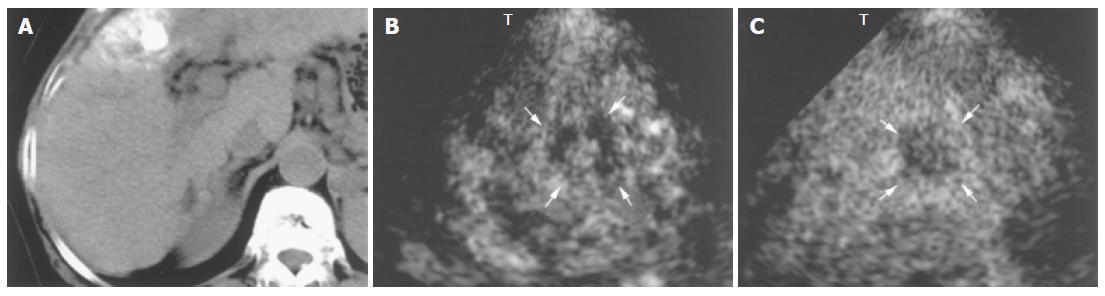
With regard to treatment, 1.5 Harmonic Imaging Sonography revealed residual blood flow signals within the tumor in 20 (47.6%) of the 42 nodules that had been treated by transcatheter arterial embolization. MRI, US CO2 angiography also revealed residual blood flow signals within the tumor, but contrast-enhanced CT demonstrated residual blood flow signals in only 14 nodules (33.3%). In the remaining 22 nodules (52.4%), no blood flow signal was observed within the tumor by 1.5 Harmonic Imaging Sonography or contrast-enhanced CT (Table 2, Figures 6 and 7).
| Findings atcontrast-enhanced CT | Vascularfindings at 1.5enhancement | Harmonic imagingsonographyno enhancement |
| Incomplete (n = 14) | 14 | 0 |
| Complete (n = 28) | 6 | 22 |
In the present study, phantom experiments were conducted to investigate the usefulness of 1.5 Harmonic Imaging Sonography. In 1.5 Harmonic Imaging Sonography, unlike 2nd harmonic imaging, images are obtained using a frequency band that is lower than that of the 2nd harmonics, resulting in a significant improvement of the contrast enhancement non-uniformity that is frequently observed when a sector transducer is used. In 1.5 Harmonic Imaging Sonography, on the other hand, since there are almost no tissue echoes, the gain can be increased. As a result, enhancement is significantly improved in deep regions from which only weak echoes are received. In addition, images are generated using the B-mode processing system, resulting in good spatial resolution and minimal motion artifacts and blooming, which are often a problem in the pseudo-Doppler method. Furthermore, the combined use of flash echo mode and monitor mode ensures that slices can be maintained or adjusted in real time even though images are acquired at high acoustic power and the microbubbles are destroyed. Therefore, the enhanced slice can easily be adjusted during the continuous injection of contrast agent. When a frequency near the mean value is used, adequate contrast enhancement is obtained in all areas, from the near field to the far field, in 1.5 Harmonic Imaging Sonography. Furthermore, this method avoids the problems associated with the Doppler method and provides extremely clear contrast-enhanced images[19,20].
The results of the present clinical study have clearly demonstrated that the use of 1.5 Harmonic Imaging Sonography is effective for evaluating the vascularity of HCCs, including the assessment of tumor vessels during continuous early-phase arterial imaging as well as the assessment of late- phase enhancement (staining) due to blood flow signals in the tumor tissues. In early-phase real-time continuous imaging, the movement of microbubbles provides the signals used to obtain enhancement. In other words, the image can depict the flow of the microbubbles in the tumor vessels. The results of our study have also shown that 1.5 Harmonic Imaging Sonography can clearly depict the hypervascular tumor vessels of HCCs. The findings obtained by late-phase imaging show partial residual enhancement of the tumor tissues, but with less enhancement than the surrounding hepatic parenchyma. It is thought that the microbubbles flow from the tumor into the hepatic parenchyma over time, resulting in lower enhancement. Residual enhancement in the area of the tumor during the late phase is believed to reflect the enhancement (staining) due to the microbubbles that are trapped in the very small, tortuous blood vessels that are characteristically found in HCCs. In the present study, however, there were six nodules (14.3%) in which the tumor vessels were identified by US CO2 angiography but not by 1.5 Harmonic Imaging Sonography. In principle, 1.5 Harmonic Imaging Sonography selectively eliminates the fundamental components in order to generate images, thus reducing the quality of B-mode images and the degree of visualization of the tumor area. In addition, the use of a sector transducer makes it difficult to assess deep regions. Generally, line density of sector scan is lower than that of convex in the deep region. Furthermore, lateral resolution of sector image is inferior to that of convex image. For these reasons, it was expected that the tumor vessels associated with small tumors in deep regions would not be detectable during the early phase. But, due to the reduction in motion artifacts, 1.5 Harmonic Imaging Sonography has significant advantages in the detection of nodules near the heart, which are difficult to detect by contrast-enhanced CT.
In the present study, the usefulness of 1.5 Harmonic Imaging Sonography and contrast -enhanced CT for evaluating treatment effects following transcatheter arterial chemoembolization of HCCs were compared, and the results showed that areas of insufficient treatment could be clearly identified by 1.5 Harmonic Imaging Sonography. Early-phase images clearly showed microbubbles flowing into the residual tumor vessels in insufficiently treated areas. Such areas were identified particularly well in late-phase images, and the information provided by such images was found to be useful for planning additional treatment. When percutaneous ethanol injection therapy and radiofrequency thermal ablation are performed under ultrasonographic guidance, 1.5 Harmonic Imaging Sonography permits viable areas within the tumor to be accurately identified during treatment and is therefore expected to improve treatment accuracy. Furthermore, 1.5 Harmonic Imaging Sonography has the advantage of being unaffected by lipiodol accumulation in the area of the tumor, which is associated with an increased risk of false-negative findings. In the present study, contrast-enhanced CT was found not to be useful for the evaluation of six nodules following transcatheter arterial chemoembolization with lipiodol.
With regard to the detection of early-stage tumor vessels, the results obtained by 1.5 Harmonic Imaging Sonography were comparable to those obtained by Sonography angiographic evaluation of blood flow signals within the tumor, except for six nodules measuring approximately 10 mm in diameter that were located 80 mm or more from the body surface. However, evaluation of all cases is difficult because of a sector probe, and an ultrasound image quality needs to be improved. The reasons for the difficulty in evaluating tumors in deep regions are thought to be (1) the use of 2.5 - and 3.0-MHz sector transducers; (2) the attenuation of ultrasound signals in deep regions due to the elimination of the fundamental components, which is characteristic of 1.5 Harmonic Imaging Sonography; and (3) MI values insufficient to destroy the microbubbles. These problems may be overcome in future when 1.5 Harmonic Imaging Sonography with the use of a convex transducer becomes available.
The next step will involve the use of a next-generation contrast agent with fluorocarbon-containing gas, which has already been employed clinically in Europe and USA. The main advantage of this contrast agent is that real-time contrast images can be obtained at low acoustic power without the need to destroy the microbubbles, and imaging techniques employing this contrast agent are therefore expected to be comparable or superior to US CO2 angiography in terms of diagnostic capabilities.
In conclusion, 1.5 Harmonic Imaging Sonography is therefore considered to be an extremely useful diagnostic imaging modality for the assessment of patients with HCC.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Choi BI, Kim TK, Han JK, Kim AY, Seong CK, Park SJ. Vascularity of hepatocellular carcinoma: assessment with contrast-enhanced second-harmonic versus conventional power Doppler US. Radiology. 2000;214:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Hosten N, Puls R, Lemke AJ, Steger W, Zendel W, Zwicker C, Felix R. Contrast-enhanced power Doppler sonography: improved detection of characteristic flow patterns in focal liver lesions. J Clin Ultrasound. 1999;27:107-115. [PubMed] [DOI] [Full Text] |
| 3. | Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, Kawasaki T, Maekawa K. Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology. 2001;221:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Heckemann RA, Cosgrove DO, Blomley MJ, Eckersley RJ, Harvey CJ, Mine Y. Liver lesions: intermittent second-harmonic gray-scale US can increase conspicuity with microbubble contrast material-early experience. Radiology. 2000;216:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Harvey CJ, Blomley MJ, Eckersley RJ, Heckemann RA, Butler-Barnes J, Cosgrove DO. Pulse-inversion mode imaging of liver specific microbubbles: improved detection of subcentimetre metastases. Lancet. 2000;355:807-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Blomley MJ, Albrecht T, Cosgrove DO, Patel N, Jayaram V, Butler-Barnes J, Eckersley RJ, Bauer A, Schlief R. Improved imaging of liver metastases with stimulated acoustic emission in the late phase of enhancement with the US contrast agent SH U 508A: early experience. Radiology. 1999;210:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Burns PN, Wilson SR, Muradali D, Microbubble destruction is the origin of Harmonic signals from FS 069. Radiology. 1996;201:158-159. |
| 8. | Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Eur J Radiol. 1998;27 Suppl 2:S157-S160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 185] [Article Influence: 6.9] [Reference Citation Analysis (34)] |
| 9. | Wilson SR, Burns PN, Muradali D, Wilson JA, Lai X. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology. 2000;215:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 213] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Blomley MJ, Albrecht T, Cosgrove DO, Eckersley RJ, Butler-Barnes J, Jayaram V, Patel N, Heckemann RA, Bauer A, Schlief R. Stimulated acoustic emission to image a late liver and spleen-specific phase of Levovist in normal volunteers and patients with and without liver disease. Ultrasound Med Biol. 1999;25:1341-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Blomley MJ, Sidhu PS, Cosgrove DO, Albrecht T, Harvey CJ, Heckemann RA, Butler-Barnes J, Eckersley RJ, Basilico R. Do different types of liver lesions differ in their uptake of the microbubble contrast agent SH U 508A in the late liver phase? Early experience. Radiology. 2001;220:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kim TK, Choi BI, Han JK, Hong HS, Park SH, Moon SG. Hepatic tumors: contrast agent-enhancement patterns with pulse-inversion harmonic US. Radiology. 2000;216:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Harvey CJ, Blomley MJ, Eckersley RJ, Cosgrove DO, Patel N, Heckemann RA, Butler-Barnes J. Hepatic malignancies: improved detection with pulse-inversion US in late phase of enhancement with SH U 508A-early experience. Radiology. 2000;216:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Yamamoto K, Shiraki K, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Murata K, Hashimoto A, Shimizu A, Okuda Y. Diagnosis of hepatocellular carcinoma using digital subtraction imaging with the contrast agent, Levovist: comparison with helical CT, digital subtraction angiography, and US angiography. Oncol Rep. 2002;9:789-792. [PubMed] |
| 15. | Yamamoto K, Shiraki K, Nakanishi S, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Murata K, Fuke H, Hashimoto A. The usefulness of digital subtraction imaging with Levovist in the diagnosis of focal hepatic tumors. Int J Oncol. 2003;22:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Kim AY, Choi BI, Kim TK, Han JK, Yun EJ, Lee KY, Han MC. Hepatocellular carcinoma: power Doppler US with a contrast agent--preliminary results. Radiology. 1998;209:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Kim TK, Han JK, Kim AY, Choi BI. Limitations of characterization of hepatic hemangiomas using a sonographic contrast agent (Levovist) and power Doppler ultrasonography. J Ultrasound Med. 1999;18:737-743. [PubMed] |
| 18. | Choi D, Lim HK, Kim SH, Lee WJ, Jang HJ, Lee JY, Paik SW, Koh KC, Lee JH. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: usefulness of power Doppler US with a microbubble contrast agent in evaluating therapeutic response-preliminary results. Radiology. 2000;217:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Tetsuya K. Technical Description of 1.5-Harmonic Imaging, an Effective Technique for Contrast-Enhanced Ultrasound Diagnosis. Medical Review. 2002;87:22-25. |
| 20. | Tetsuya K, Yoshitaka M, Naohisa . New Imaging Method for High MI Contrast Imaging: 1.5 Harmonic Imaging. J Ultra-sound Med. 2002;29:241. |
| 21. | Kudo M, Tomita S, Tochio H, Kashida H, Hirasa M, Todo A. Hepatic focal nodular hyperplasia: specific findings at dynamic contrast-enhanced US with carbon dioxide microbubbles. Radiology. 1991;179:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Kudo M, Tomita S, Tochio H, Mimura J, Okabe Y, Kashida H, Hirasa M, Ibuki Y, Todo A. Small hepatocellular carcinoma: diagnosis with US angiography with intraarterial CO2 microbubbles. Radiology. 1992;182:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (0)] |