Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5577
Revised: March 20, 2005
Accepted: March 23, 2005
Published online: September 21, 2005
A 62-year-old male was referred to our hospital because of liver dysfunction, diffuse pancreatic swelling, and trachelophyma. At admission, the patient was free of pain. Physical examination showed enlarged and palpable bilateral submandibular masses, but no palpable mass or organomegaly in the abdomen. Laboratory findings were as follows: total protein 90 g/L with γ-globulin of 37.3% (33 g/L), total bilirubin 4 mg/L, aspartate aminotransferase 39 IU/L, alanine aminotransferase 67 IU/L, γ-glutamyl transpeptidase 1 647 IU/L, and amylase 135 IU/L. Autoanti-bodies were negative, and tumor markers were within the normal range. Serum IgG4 level was markedly elevated (18 900 mg/L). Computed tomography (CT) showed diffuse swelling of the pancreas and dilatation of both common and intra-hepatic bile ducts. Endoscopic retrograde pancreatography (ERP) revealed diffuse irregular and narrow main pancreatic duct and stenosis of the lower common bile duct. Biopsy specimens from the pancreas, salivary gland and liver showed marked periductal IgG4-positive plasma cell infiltration with fibrosis. We considered this patient to be autoimmune pancreatitis (AIP) with fibrosclerosis of the salivary gland and biliary tract, prescribed prednisolone at an initial dose of 40 mg/d. Three months later, the laboratory data improved almost to normal. Abdominal CT reflected prominent improvement in the pancreatic lesion. Swelling of the salivary gland also improved. At present, the patient is on 10 mg/d of prednisolone without recurrence of the pancreatitis. We present here a case of AIP with fibrosclerosis of salivary gland and biliary tract.
- Citation: Taguchi M, Aridome G, Abe S, Kume K, Tashiro M, Yamamoto M, Kihara Y, Nakamura H, Otsuki M. Autoimmune pancreatitis with IgG4-positive plasma cell infiltration in salivary glands and biliary tract. World J Gastroenterol 2005; 11(35): 5577-5581
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5577.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5577
A 62-year-old Japanese male was referred to our hospital because of liver dysfunction, common and intra-hepatic bile duct dilatation, diffuse pancreatic swelling, and trachelophyma. He had complained about fatigability and appetite loss without abdominal pain and noticed enlarged bilateral submandibular masses. There was no past history of pancreatitis, biliary tract disease or collagen disease. He was not a habitual drinker, and his family history was not contributory.
On admission, the patient was free of pain. Physical examination showed enlarged and palpable bilateral submandibular masses, but no palpable mass or organomegaly in the abdomen. Laboratory tests showed elevation of hepatobiliary enzyme levels without hyperbilirubinemia: total-bilirubin 7 mg/L, aspartate aminotransferase 39 IU/L, alanine aminotransferase 67 IU/L, alkaline phosphatase 1 293 IU/L, γ-glutamyl transpeptidase 1 647 IU/L. BUN and creatinine (Cre) levels were also elevated; BUN 230 mg/L, Cre 17 mg/L. Serum IgG level was elevated to 33 680 mg/L, serum IgG4, a subclass of IgG, was especially elevated to 1 890 mg/mL, although autoantibodies such as anti-nuclear antibodies (ANA), rheumatoid factor (RF), SS-A and SS-B antibody were negative. Pancreatic enzyme levels were elevated except for amylase (Amy); Amy 135 IU/L, lipase 95 IU/L, trypsin 584 ng/mL, elastase I 5 200 ng/L, and pancreatic exocrine function determined by the urinary para-aminobutyric acid excretion rate (BT-PABA test) was 27.5%. Tumor markers were also elevated: CA19-9 125 U/mL, DUPAN-2 330 U/mL, and SPAN-1 97 U/mL. The 75 g oral glucose tolerance test showed a diabetic pattern. The glucagon-loading test revealed impaired insulin secretory function.
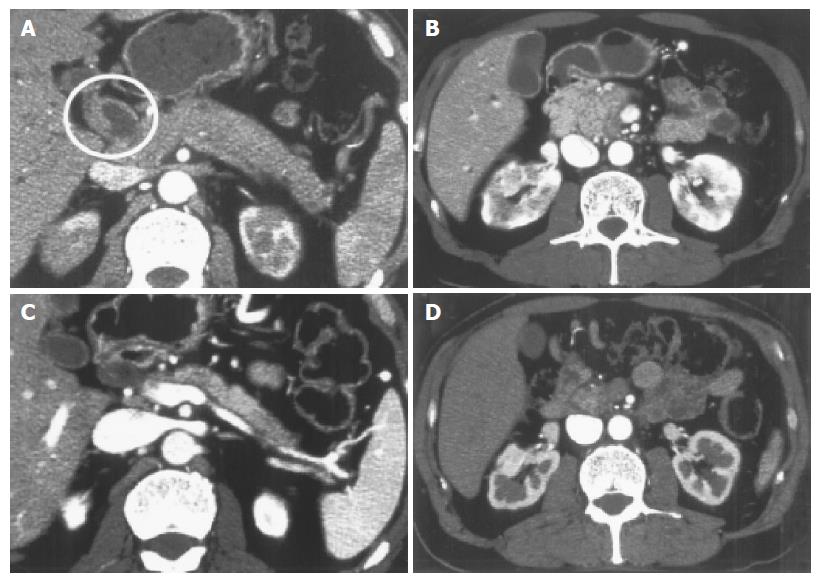
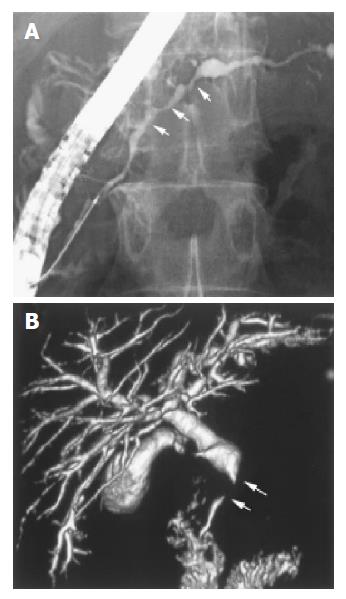
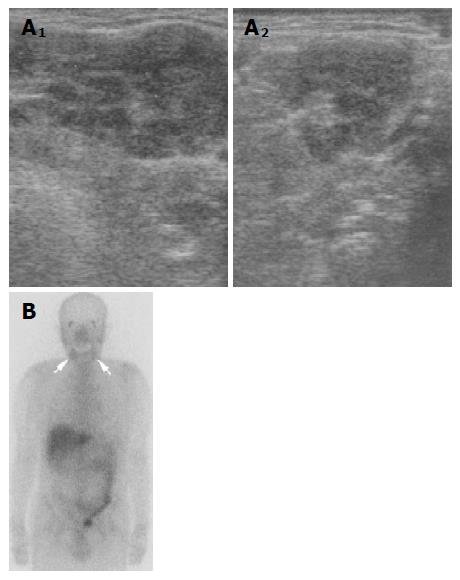
Contrast-enhanced abdominal CT demonstrated diffuse enlargement of the pancreas, wall thickness of the dilated common bile duct, and heterogeneous enhancement of both kidneys (Figures 1A and B). Endoscopic retrograde pancreatography (ERP) revealed an irregular narrowing of the entire main pancreatic duct (Figure 2A). Drip infusion cholangiography-CT (DIC-CT) showed a smooth stenosis of the lower common bile duct with upstream dilatation (Figure 2B). Ultrasonography (US) of the neck showed diffuse swelling of bilateral salivary glands and Ga scintigraphy revealed abnormal uptake in the salivary glands (Figures 3A and B).
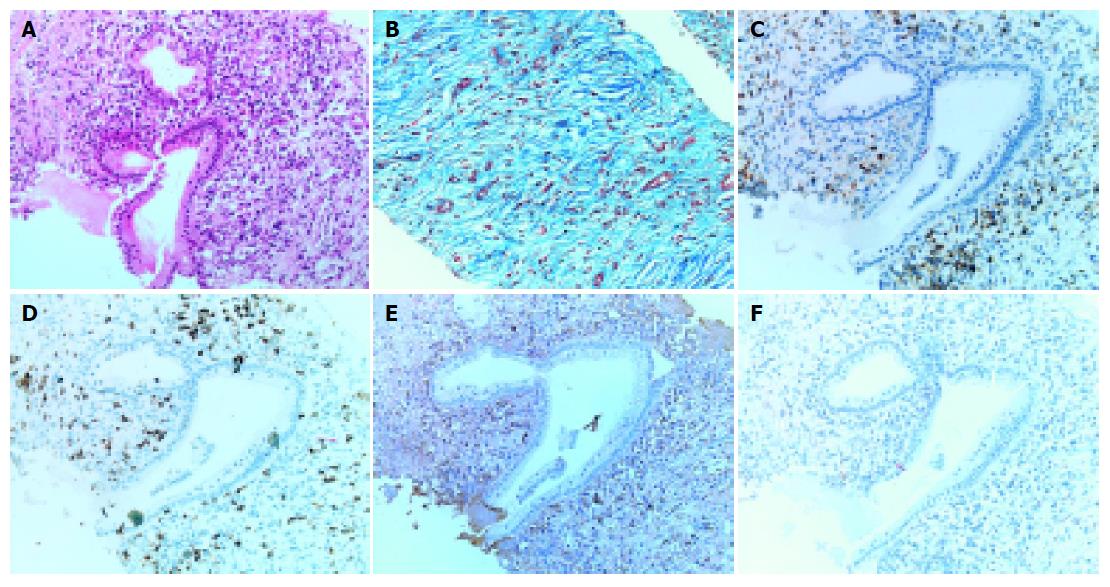
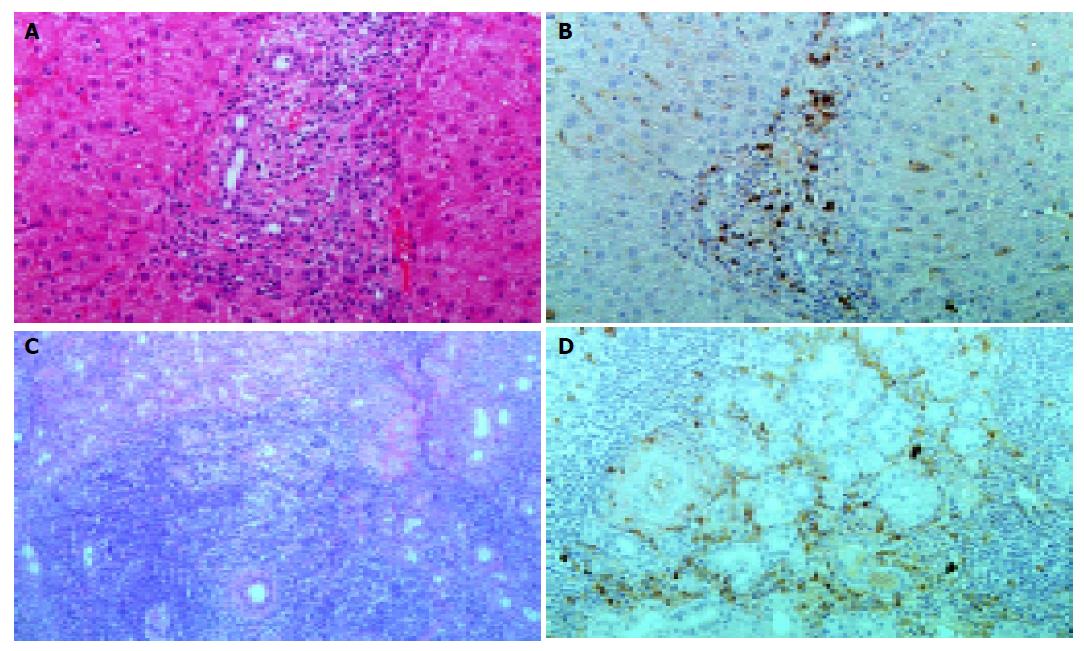
Pancreatic tissue samples obtained by needle biopsy under US showed periductal lymphoplasmacytic infiltration and marked interstitial fibrosis with acinar atrophy (Figures 4A and B). Immunohistochemically, diffuse infiltrates in the pancreas consisted predominantly of CD4- or CD8-positive T lymphocytes, and IgG4-positive plasma cells rather than CD20-positive B lymphocytes (Figures 4C-F). Liver and salivary gland tissue samples by needle biopsy also showed the periductal IgG4-positive plasma cell infiltration with interstitial fibrosis (Figures 5A-D).
Oral administration of prednisolone at an initial dose of 40 mg/d, following a diagnosis of autoimmune pancreatitis (AIP), led to an immediate improvement in all blood chemistries including tumor markers and serum γ-globulin concentration. In addition, the endocrine function improved from HbA1c 6.6% to 5.7%, the exocrine function determined by BT-PABA test also improved from 27.5% to 52.9% and renal function determined by serum Cre improved from 17 to 11 mg/L after steroid therapy. Subsequent follow-up CT showed improvement in pancreatic swelling, stenosis of lower common bile duct with upstream dilatation and wall thickness of the bile ducts and heterogeneous enhancement of both kidneys (Figures 1C and D). Moreover, the salivary glands swelling also improved. Prednisolone was gradually reduced to 10 mg/d over the course of 9 wk without any exacerbation.
AIP is a recently noted clinical entity and a rare group of pancreatitis in which autoimmunity is a suspected pathogenic mechanism. The diagnostic criteria for AIP according to the Japan Pancreas Society (2002) are as follows: (1) Diffuse or segmental narrowing of the main pancreatic duct with irregular wall (more than 1/3 length of the entire pancreas) and diffuse or localized enlargement of the pancreas by imaging studies, such as US, CT and magnetic resonance imaging. (2) High serum γ-globulin and/or IgG, or the presence of autoantibodies, such as ANA and RF. (3) Marked inter-lobular fibrosis and prominent infiltration of lymphocytes and plasma cells in the periductal area occasionally with lymphoid follicles in the pancreas. Diagnosis of AIP is established when criterion 1, together with criteria 2 and/or 3, are fulfilled[1-3]. We diagnosed our patient as AIP by these criteria.
Recent studies have revealed extrapancreatic involvements in AIP, such as sclerosing cholangitis, retroperitoneal fibrosis, and salivary gland swelling[4,5]. In AIP patients, the high serum levels of IgG4, subclass of IgG, and IgG4-positive lymphoplasmacytic infiltration are observed not only in the pancreas, but also in extra-pancreatic organs, such as the biliary duct, salivary gland and lymph nodes. These observations suggest a common pathogenesis related to the IgG4 subclass of immune complexes[6,7]. In our case, immunohistochemical examination of biopsied specimens showed infiltration of IgG4-positive plasma cells in the periductal area of the salivary gland (Figure 5D) and the portal area of the liver (Figure 5B), as well as in the pancreas (Figure 4E).
Nishimori et al[8], found jaundice in 30% of AIP patients and stenosis of the lower common bile duct in 68% of 64 AIP patients. Two possible mechanisms of stenosis were hypothesized: one is the direct effect of the swollen pancreas on the bile duct, the other is wall inflammatory swelling of the bile duct wall, independent of the pancreas. In our case, smooth stenosis of the lower common bile duct was accompanied by wall thickening of bile duct (Figure 1A), which improved completely after steroid therapy (Figure 1C). These results support the latter hypothesis. It is reported that the histological features of bile duct lesions are similar to those typically seen in the pancreas[9]. Moreover, the liver biopsy specimen showed IgG4-positive cell infiltration around the small bile duct in the portal area (Figure 5B). In fact, the focus of the bile duct, one of the extrapancreatic lesion of AIP, is diffuse from the common to intrahepatic bile ducts. Stenosis of the upper common bile was seen in 53% of AIP patients[8].
Pancreatic islets as well as acinar cells are involved in the same inflammatory process in AIP[10]. Kamisawa et al[11], reported that 42% and 88% of AIP showed pancreatic endocrine and exocrine dysfunction, respectively. The endocrine and exocrine function improved in 60% and 50% of these patients after steroid therapy, respectively. Although we were unable to demonstrate extensive islet destruction in the pancreas due to lack of islets in the biopsy specimen, HbA1c, a marker of glycemic control, improved without anti-diabetic treatment and the exocrine function evaluated by BT-PABA test also improved after steroid therapy.
Salivary gland function, which is known to be frequently impaired in patient with AIP, even when clinical symptoms are not obvious as determined by β2-microglobulin level in the saliva and parotid gland scintigraphy improves after steroid therapy in almost all AIP patients[11,12]. In our case, the patient noticed swelling of the salivary glands and abundant infiltration of IgG4-positive plasma cells, which has not been detected in Sjögren’s syndrome[7]. In addition, specific marker of Sjögren’s syndrome, anti SS-A and SS-B antibodies were negative. Swelling of the salivary glands disappeared after steroid therapy. These results suggest that the salivary swelling was due to extrapancreatic lesion of AIP rather than due to Sjögren’s syndrome. Ductal system of salivary gland and pancreas are similar in histological structure, so common autoantigen might be involved in the pathogenesis of these organs.
On admission, mild renal dysfunction was seen and contrast-enhanced CT demonstrated heterogeneous enhancement of both kidneys (Figure 1B). Treatment with steroid improved CT findings (Figure 1D) and renal function. Renal dysfunction in AIP is reported to be due to retroperitoneal fibrosis[13] and/or IgG4-associated tubulointerstitial nephritis[14,15]. Although we could not prove histologically, renal dysfunction in the present case was probably due to tubulointerstitial nephritis, one of the extrapancreatic involvements in AIP.
Following steroid therapy, most of all clinical symptoms and laboratory data improved and the pancreas became small and atrophic (Figure 1C). Takayama et al[16], reported pancreatic stones in 20% of AIP patients during observation for 4.5 years. This suggests that AIP is a progressive disease and a possibility of an early stage of idiopathic chronic pancreatitis. We continued maintenance steroid therapy at 10 mg/d, but prognosis with AIP is also unknown at this stage.
In conclusion, we presented here a case of AIP with IgG4-associated fibrosclerosis of the salivary gland and biliary tract. AIP may be one lesion of systemic multifocal fibrosclerosis in some cases.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Members of the criteria committee for autoimmune pancreatitis of the Japan pancreatic society. Diagnostic criteria for autoimmune pancreatitis by the Japan pancreas society. J Jpn Panc Soc. 2002;17:585-587. |
| 2. | Otsuki M. Chronic pancreatitis in Japan: epidemiology, prognosis, diagnostic criteria, and future problems. J Gastroenterol. 2003;38:315-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Otsuki M. Chronic pancreatitis. The problems of diagnostic criteria. Pancreatology. 2004;4:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Kawa S, Hamano H. Autoimmune pancreatitis and bile duct lesions. J Gastroenterol. 2003;38:1201-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 1878] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Nishimori I, Suda K, Oi I. Research on the clinical state of autoimmune pancreatitis. J Jpn Panc Soc. 2002;17:619-627. |
| 9. | Kuroiwa T, Suda T, Takahashi T, Hirono H, Natsui M, Motoyama H, Nomoto M, Aoyagi Y. Bile duct involvement in a case of autoimmune pancreatitis successfully treated with an oral steroid. Dig Dis Sci. 2002;47:1810-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Tanaka S, Kobayashi T, Nakanishi K, Okubo M, Murase T, Hashimoto M, Takeuchi K. Corticosteroid-responsive diabetes mellitus associated with autoimmune pancreatitis. Lancet. 2000;356:910-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kamisawa T, Egawa N, Inokuma S, Tsuruta K, Okamoto A, Kamata N, Nakamura T, Matsukawa M. Pancreatic endocrine and exocrine function and salivary gland function in autoimmune pancreatitis before and after steroid therapy. Pancreas. 2003;27:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Kamisawa T, Tu Y, Egawa N, Sakaki N, Inokuma S, Kamata N. Salivary gland involvement in chronic pancreatitis of various etiologies. Am J Gastroenterol. 2003;98:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Uchida K, Okazaki K, Asada M, Yazumi S, Ohana M, Chiba T, Inoue T. Case of chronic pancreatitis involving an autoimmune mechanism that extended to retroperitoneal fibrosis. Pancreas. 2003;26:92-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Takeda S, Haratake J, Kasai T, Takaeda C, Takazakura E. IgG4-associated idiopathic tubulointerstitial nephritis complicating autoimmune pancreatitis. Nephrol Dial Transplant. 2004;19:474-476. [RCA] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Uchiyama-Tanaka Y, Mori Y, Kimura T, Sonomura K, Umemura S, Kishimoto N, Nose A, Tokoro T, Kijima Y, Yamahara H. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. Am J Kidney Dis. 2004;43:e18-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Takayama M, Hamano H, Ochi Y, Saegusa H, Komatsu K, Muraki T, Arakura N, Imai Y, Hasebe O, Kawa S. Recurrent attacks of autoimmune pancreatitis result in pancreatic stone formation. Am J Gastroenterol. 2004;99:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |