CASE REPORT
A 65-year-old Caucasian female came to the emergency department of our hospital complaining of bloody diarrhea. Twelve hours before admission the patient experienced a dull abdominal pain of sudden onset, located at the hypogastrium, and her temperature rose to 37.8 °C. She reported 10 bowel movements, which were diarrheal at first and became hemorrhagic later. Physical examination revealed moderate tenderness on palpation of the hypogastrium and increased bowel sounds on auscultation. Inspection of the skin revealed two firm, subcutaneous masses with ill-defined borders, each one located at the apex of the scapula with a diameter of 2 cm. Physical examination of the lungs, heart, skin, and other body systems revealed no abnormal features. It was the first episode of bloody diarrhea experienced by the patient. Her past medical history included essential hypertension, hyperlipidemia, and an acute coronary syndrome and she was receiving an angiotensin-converting enzyme inhibitor, enteric-coated aspirin and a statin. Furthermore, she had atopy to grass, tree pollens and fungal spores, manifested by bronchoconstriction. There was no family history of intestinal diseases.
Laboratory investigation including complete blood count, biochemical examination, tumor markers (CEA, CA 19-9, aFP, CA125, CA 15-3), coagulation tests, microbiological cultures for bacterial and parasites in feces and cultures of the urine were normal except for a slightly elevated blood glucose level (120 mg%) and CRP (12, normal <4). Chest x-ray and ECG had no abnormal features. An abdominal plain radiograph showed only an air-filled large bowel.
Colonoscopy was performed after cessation of the hemorrhage. The endoscope reached the hepatic flexure and revealed a segmental colitis. At the splenic flexure and proximal portion of the descending colon there were erythema and edema of the mucosa with hemorrhagic petechiae. A sessile polyp 1 cm in diameter was detected at the descending colon, near the site of inflammation and was endoscopically removed. The transverse colon and the rectum were macroscopically normal. At the sigmoid colon a few, small diverticulae were noticed, without inflammation. Ultrasonography of the abdomen revealed no pathological findings.
Pathology findings
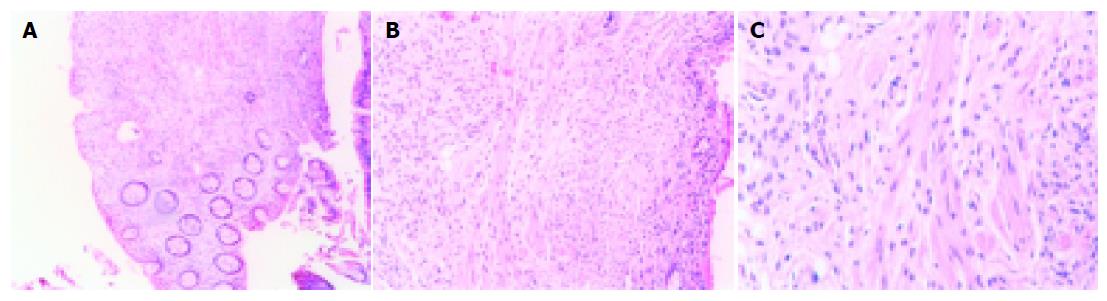
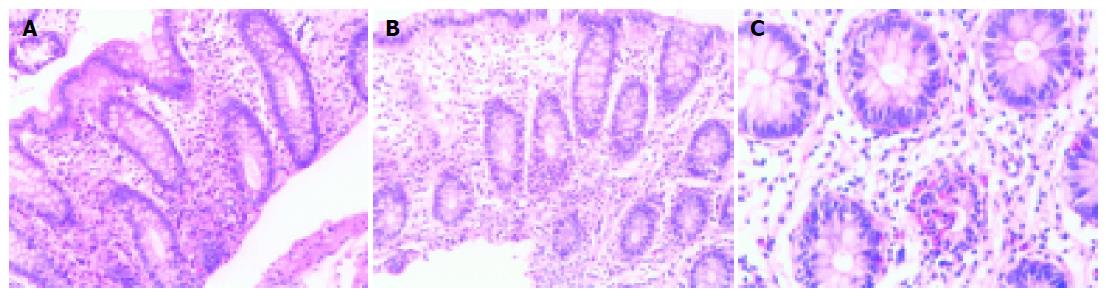
Microscopically, the biopsy from the polyp showed fragments of mucosa of the large bowel lined by normal epithelium. In the lamina propria there was a nodule, with ill-defined borders, which was composed of longitudinal cells with elongated, pointed nucleus. These cells were deposited in interlaced bundles and in near proximity with collagen fibers. There were no signs of atypia, nor significant mitotic activity. Immunohistochemical stains were positive for vimentin and S-100 protein but negative for desmin, smooth muscle actin, c-kit and CD34. The above morphological and immunohistochemical characteristics were consistent with the diagnosis of neurofibroma (Figures 1A-C). Biopsy samples from the site of colitis showed focal erosions of the surface mucosal epithelium and focal decrease in mucus production. The architecture of the glands was intact. There was edema and dense infiltrate of inflammatory cells (lymphocytes, polymorphonuclear and plasma cells) in the lamina propria. There was an increased number of eosinophils in the same area. Crypt abscesses were rarely encountered. Granulomas were not recognized microscopically. These findings could not support a definite diagnosis of idiopathic inflammatory bowel disease and were classified as nonspecific colitis (Figures 2A-C).
Figure 1 A: Neurofibroma, hematoxylin-eosin (H-E) ×100; B: Neurofibroma, H-E ×250; C: Neurofibroma, H-E ×400.
Figure 2 A: Nonspecific colitis, H–E ×250; B: Nonspecific colitis, H-E ×250; C: Nonspecific colitis, crypt abscess, H-E ×400.
Clinically the patient had no caf-au-lait spots on her skin, axillary freckling or peripheral neurofibromas. Also there were no pigmented hamartomas of the iris and no family history of neurofibromatosis. The patient received crystalloid solutions, antibiotics and mesalazine, had an uneventful recovery and was discharged 5 d later. Two months later in a follow-up examination, the patient had no symptoms from the intestine and her physical examination was unremarkable. Colonoscopy was again performed and the colon was examined to the hepatic flexure. The previous findings were absent and the mucosal surface was normal. The patient was advised to have a barium enema done for the visualization of the remaining large bowel which showed only diverticula of the right colon and sigmoid. Due to the polypoid neurofibroma the patient underwent spiral CT of the brain, thorax and abdomen for pathological features associated with neurofibromatosis. No abnormalities were found. Biopsy samples, taken from one of the subcutaneous masses located at the back of the patient showed a lipoma with fibrous components and S-100 negative immunoreactivity. New serum laboratory tests were normal except glucose, which was 142 mg%. Gastroscopy revealed erosive duodenitis and no other suspicious pathologic lesion.
DISCUSSION
Neurofibromas are usually manifestations of the von Recklinghausen’s disease. They are benign neoplasms consisting of neural and connective tissue components like Schwann and perineural cells and myofibroblasts. They are usually multiple on presentation and are part of a hereditary disorder with two clinical forms: Nf1 (or von Recklinghausen’s neurofibromatosis or peripheral neurofibromatosis, Nf1) and neurofibromatosis type 2 (or central neurofibromatosis or bilateral acoustic neurofibromatosis). These disease entities have variable clinical expressions with manifestations involving the skin, nervous system, eyes, bones, gastrointestinal tract, and other body parts. Cardiovascular disease manifested as hypertension, myocardial infarction, hemorrhage, or cerebral ischemia, are the most frequent causes of death in Nf1 patients[3]. Survival is lesser than expected in the normal population as much as 15 years. There is also a disease type called segmental neurofibromatosis, which involves a specific body organ without the variety of multiple pathologic expressions of the generalized form.
Neurofibromas of the digestive system are usually found as part of the Nf1. This is a relative common inherited disorder based on genetic transmission of the autosomal dominant type. The incidence of the disease is 1:3 000 births and the population prevalence is 1:5 000[1,4]. The diagnostic criteria for Nf1 include the following: (1) the so-called “café-au-lait” spots (at least six spots, size ≥1.5 cm in postpubertal patients), (2) the characteristic neurofibromas (at least two or one plexiform), (3) axillary or groin freckling, (4) pigmented hamartomas of the iris, the so-called Lisch nodules (at least two), (5) an osseous lesion which may include thinning of the long bone cortex or sphenoid wing dysplasia, (6) first degree relative with Nf1, (7) optic glioma. The patient should have at least two of the previous criteria to be considered as Nf1. The penetrance of the disease is 100% until age 5[5]. The majority of patients have caf-au-lait spots (95%), neurofibromas (95%), Lisch nodules (95%) and axillary freckling (70%). Optic gliomas affect 15% of the patients[1]. Café-au-lait spots most commonly present before 2 years of age[6].
Neurofibromas may be cutaneous, nodular derived from peripheral nerves or plexiform with aggressive, infiltrative growth that extends outside the nerve sheath to the surrounding tissue. Although benign, neurofibromas of the nodular and particular the plexiform type may demonstrate a malignant transformation in 2-16% of affected individuals[1,2,7]. About 50% of the cases of Nf1 are not familial but sporadic as a result of germ-cell mutations. Segmental Nf seems to involve a group of somatic cells that have exclusively acquired the specific neurofibromatosis mutations presenting a mosaicism[4]. The Nf1 gene has been located on chromosome 17q11.2 and produces a peptide of 2 818 aminoacids, called neurofibromin, which works as a tumor suppressor[1,3]. Neurofibromin seems to interact with the RAS/GTP membrane-signaling pathway in cells by activating the GTPase that converts GTP to inactive GDP and therefore shutting down the RAS effector system which is involved in cell growth and differentiation. Mutations of the Nf1 gene result in deficient neurofibromin, which in turn seems unable to control responses to proliferation signaling thus giving birth to neoplastic growth.
Participation of the gastrointestinal tract has been documented in 25% of patients with Nf1. Ganglioneuromatosis and neurofibromatosis are the pathologic forms of gastrointestinal involvement[8]. Ganglioneuromatosis refers to extended hyperplasia and hypertrophy of the nerve plexuses and ganglion cells in the mucosa or throughout the intestinal wall. Characteristic neurofibromas have been found in the digestive tract in 11% of patients with Nf1[5].
Intestinal neurofibromatosis exists, in the majority of cases, in association with Nf1 and only rarely presents outside this disease as a separate pathologic entity (familial or sporadic)[2]. Multiple neurofibromas are discovered in the gut, more often located in the jejunum, stomach, ileum, duodenum, and colon according to the frequency of their appearance[1,5]. Neurofibromas are usually originating from either the plexus of Meissner in the submucosa or the plexus of Auerbach’s in the muscularis propria or even from the serosa[2,9]. The lesions are often sessile and wide based but also pedunculated polyps have been observed[2]. These lesions most often are discovered in the 4th-6th decade of life[8-10]. It is common to remain clinically silent and rarely cause symptoms before puberty. Nf1 is also associated with the occurrence of other neoplasms that involve the gastrointestinal tract. These include carcinoids and somatostatinomas that are located mainly in the duodenum and ampulla of Vater, stromal tumors mainly leiomyomas and sarcomas and pancreatic adenocarcinomas[5,8,10]. The clinical picture includes abdominal pain, palpable masses, hemorrhage due to necrosis or ulceration of the mucosa, obstruction due to intussusception or extraluminar pressure, perforation, megacolon, peptic ulcer disease, diarrhea, steatorrhea, obstructive jaundice and obstruction of the pancreatic tract. Iron deficiency anemia has also been reported and caused by occult blood loss[2,5,11].
Neurofibromatosis of the colon as part of the Nf1 is rare[7,9,12-14]. Isolated colonic neurofibromatosis without other features suggestive of Nf1 has been very rarely encountered in the clinical practice. Only 13 such cases of isolated colonic neurofibromatosis with no evidence of Nf1 have been documented in the literature[2,15]. Isolated neurofibromas of the colon are even rarer and only two such cases have been published[11]. In the first case an asymptomatic polyp was discovered to represent a neurofibroma while in the second case the neurofibroma presented as a large polypoid mass in the transverse colon. The mass had intussuscepted into the rectum causing massive bleeding of the gastrointestinal tract. There are also other parts of the digestive tract in which solitary neurofibromas have been detected. There have been 8 cases of solitary mesentery neurofibroma (7 ileal, 1 gastrocolic)[6], 1 case of ileal isolated neurofibroma[16], 3 cases of the anal canal[17], 1 case of the esophagus[18], 1 case of the soft palate[19], 7 cases of the gallbladder[20], and 1 case of the common bile duct[21].
Isolated neurofibromas in other body organs are also very rare. Very few cases have been reported so far, namely to the kidney[22], spermatic cord[23], nasal cavity[24], palatine tonsil[25], parapharyngeal space[26], larynx[27], humerus[28], submandibular salivary gland[29], conjuctiva[30], retroperitoneal space[31], cranial ventricles[32], and chest wall[33].
In our case a solitary neurofibroma was discovered in the descending colon in the form of a sessile polyp. The patient has no associated features of von Recklinghausen’s disease. The patient presented with lower gastrointestinal bleeding. In colonoscopy a segmental colitis was revealed, involving the splenic flexure and associated proximal part of the descending colon. Although a definite diagnosis of ischemic colitis cannot be made, there are many clues that support this explanation. The medical history of atherosclerotic disease including hypertension, ischemic heart attack, diabetes and hyperlipidemia (syndrome X), which justify the presence of predisposing factors for the development of vascular blood flow defects as a pathogenetic mechanism for ischemic attacks. The age of the patient is compatible with ischemia. The symptoms and physical findings are among those found in ischemic, perfusion-compromised bowel: sudden onset of pain in the lower abdomen, passage of red blood with stools within 24 h and abdominal tenderness. The endoscopic findings of transient, segmental colitis located around the splenic flexure are a characteristic pattern in 11% of colonic ischemia. The histologic evaluation of the biopsy specimens revealed a nonspecific inflammatory process, which is mostly encountered in mild cases of ischemic colitis. The clinical course with remission of symptoms within 72 h and the reversal of mucosal damage after 8 wk correlates with the usual prognosis of mild ischemic colitis. In less than 10% of patients with colonic ischemia, there is a potentially obstructing lesion located distally to the ischemic segment with normal intervening mucosa between them. In our case the polypoid neurofibroma and the diverticula of the sigmoid appear at a site distal to the colitis.
Aspirin, which is a drug implicated in the induction of inflammatory colitis, might have attributed to the patient’s symptoms. In a recent review, 5 cases of inflammatory colitis have been attributed to hypersensitivity reactions caused by salicylates[34]. The clinical presentation in these cases has included, among others, abdominal pain, distention, and pyrexia. Endoscopy has revealed erythema, friability and ulcers and the histologic examination included an eosinophilic infiltration and crypt abscesses. Many of the above symptoms and signs also exist in our case and therefore aspirin colitis may also be an explanation for our findings.