Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5521
Revised: February 15, 2005
Accepted: February 18, 2005
Published online: September 21, 2005
AIM: To investigate if and to what extent antiviral therapy influenced a broad panel of quantitative testing of liver function (QTLF).
METHODS: Fifty patients with chronic hepatitis C were either treated with interferon (n = 8), interferon/ribavirin (n = 19) or peg-interferon/ribavirin (n = 23). Quantitative testing of liver function, including aminopyrine breath test (ABT), galactose elimination capacity (GEC), sorbitol clearance (SCl) and indocyanine green clearance (ICG) was performed before and 3 mo after initiation of antiviral therapy.
RESULTS: After 3 mo of antiviral treatment, 36 patients showed normal transaminases and were negative for HCV-RNA, 14 patients did not respond to therapy. ABT and GEC as parameters of microsomal and cytosolic liver function were reduced in all patients before therapy initiation and returned to normal values in the 36 therapy responders after 3 mo. Parameters of liver perfusion (SCl and ICG) were not affected by antiviral therapy. In the 14 non-responders, no changes in QTLF values were observed during the treatment period.
CONCLUSION: ICG and SCl remained unaffected in patients with chronic hepatitis C, while ABT and GEC were significantly compromised. ABT and GEC normalized in responders to antiviral therapy. Early determination of ABT and GEC may differentiate responders from non-responders to antiviral treatment in hepatitis C.
- Citation: Ocker M, Ganslmayer M, Zopf S, Gahr S, Janson C, Hahn EG, Herold C. Improvement of quantitative testing of liver function in patients with chronic hepatitis C after installment of antiviral therapy. World J Gastroenterol 2005; 11(35): 5521-5524
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5521.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5521
Chronic infection with HCV is among the leading causes for impaired liver function and a major risk factor for developing liver cirrhosis and subsequently hepatocellular carcinoma[1,2]. Treatment of patients with chronic HCV infection has significantly improved, since the introduction of INF-based therapies[3-6]. Yet, more than 50% of patients infected with HCV genotype 1 and approximately 20% of patients with genotypes 2 and 3 do not achieve sustained virologic responses. Risk factors for non-response are HCV genotype, HCV RNA level and liver cirrhosis, which is usually accompanied by impaired liver function and blood flow[7]. Stage and prognosis of liver cirrhosis can be assessed by clinical examination, conventional blood tests and ultrasound or computed tomography. Yet, these conventional parameters (e.g., serum albumin, cholinesterase or coagulation factors) often lack sensitivity in early stages of liver diseases and are only changed in advanced stages. Therefore QTLF, which are based on the hepatic clearance or metabolism of test substances, have been successfully used to predict prognosis and outcome of a variety of different liver diseases[8-14].
So far, response to antiviral therapy is mainly based on HCV RNA levels and transaminases. Here, we investigated if and to what extent changes in either hepatic metabolic enzyme function and in liver perfusion can be achieved by different antiviral therapies, and if changes in QTLF may help to discriminate responders from non-responders to antiviral treatment strategies.
Fifty consecutive patients (age 39±13 years) with chronic hepatitis C (HCV-RNA positive and raised transaminases, mild to moderate fibrosis, all with HCV genotype 1) were included in the study. Eight patients (16%) were treated with INF monotherapy, 19 patients (38%) with INF/RIB and 23 patients (46%) with peg-INF/RIB combination therapy. All patients underwent QTLF before and 3 mo after initiation of antiviral therapy. Drugs that could possibly interfere with QTLF were discontinued 120 h before testing. Twenty-five healthy adults with normal hepatic serum parameters served as controls[8].
HCV-RNA was assessed qualitatively and quantitatively using the HCV Monitor and the Cobas Amplicor systems, respectively (both from Roche, Germany). Response to antiviral treatment was defined as being negative for qualitative HCV testing or less than two log steps drop in quantitative HCV-RNA levels.
The following tests were performed to quantitatively assess changes in liver function during antiviral therapy: aminopyrine breath test (ABT), galactose elimination capacity (GEC), sorbitol clearance (SCl) and indocyanine green clearance (ICG). ABT measures the metabolic capacity of hepatocytes by determining the activity of microsomal enzymes[15]. In detail, radioactively labeled 14C-aminopyrine (1.5 μCr), applied intravenously, is demethylated by amino-N-demethylase, which reflects the activity of three different cytochrome P450-dependent microsomal enzymes, and further metabolized to 14CO2 which is exhaled and measured with a β-counter.
GEC determines the cytosolic metabolic capacity of the liver[16]. The catabolic enzyme system is saturated by an intravenous bolus injection of galactose (45% galactose, 0.5 g/kg body weight). The cytoplasmic enzyme galactokinase phosphorylates galactose determines the elimination of this hexose, serving as a measure of functional liver cell mass. Serum concentrations of galactose are determined photometrically at 366 nm at 0 min and from 20 to 60 min p.i. in 5 min intervals.
SCl is regarded as a parameter of liver parenchymal perfusion (liver plasma flow). Sorbitol (500 g/L) is administered via a perfusor at 7.5 mL/h. The hepatic clearance corresponds to the measurement of the arteriovenous concentration difference[17]. Serum and urinary concentrations of sorbitol are determined photometrically at the beginning of the perfusion and after reaching steady-state conditions (150 min).
ICG is exclusively absorbed in the liver and quickly excreted in unmodified form (97%) in the bile and can therefore be used as a marker for liver perfusion and biliary secretion capacity after infusion of a bolus of 0.5 mg/kg body weight. Its serum concentration is measured photometrically at 800 nm in 3 min intervals until 21 min after ICG injection[18].
Transaminase levels (AST, ALT) were determined by routine methods in the Department of Medical Chemistry, University Erlangen.
Statistical analysis was performed using SPSS for Windows with P<0.05 regarded as significant. Spearman’s correlation coefficient (r) was calculated with Microsoft Excel 2003 for Windows XP. The present study was performed in concordance with the ethical standards formulated in the version of 1964 Declaration of Helsinki. All patients gave their informed consent prior to inclusion in the study.
Of the 50 treated patients, 36 (72%) responded to the respective antiviral therapy, showing normal transaminases and negative HCV-RNA after 3 mo. Fourteen patients (28%) did not respond to the therapy regimens (Table 1). Transaminase levels correlated well with HCV-RNA copy numbers, with r = 0.966 and r = 0.937 for AST and ALT, respectively.
| HCV patients (n = 50) | Responders (n = 36) | Non-responders (n = 14) | Controls (n = 25) | |
| Age (yr) | 39±13 | 41±12 | 37±11 | 39±10 |
| Sex (m/f) | 33/17 | 25/11 | 10/4 | 17/8 |
| AST (U/L) | 74±37 | 35±13 | 59±17 | 27±11 |
| ALT (U/L) | 97±45 | 30±11 | 63±21 | 23±13 |
| RNA level (×1 000) | 723±234 | Neg/2 log drop | 649±305 | Negative |
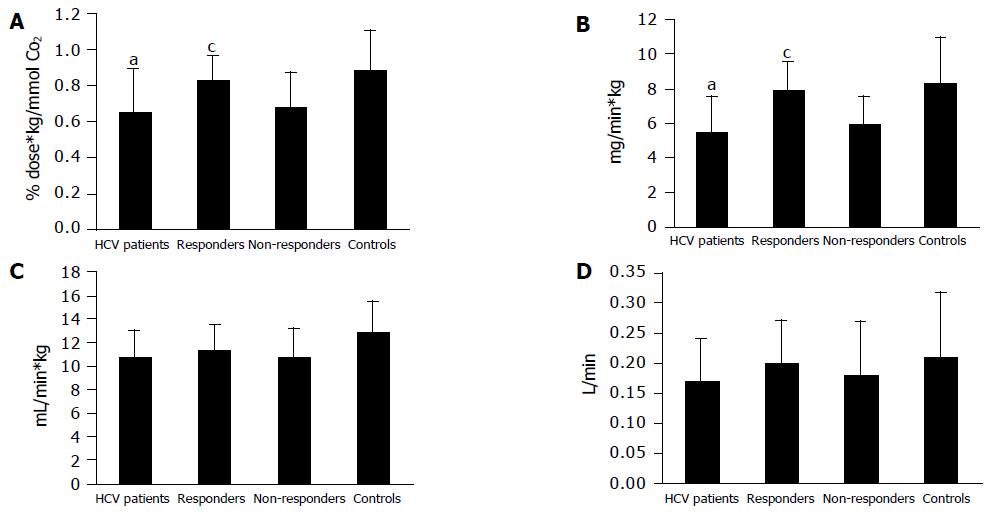
ABT (Figure 1A) and GEC (Figure 1B) were reduced in all patients before beginning the treatment (ABT: 0.65±0.24% dose-kg/mmol CO2; GEC: 5.43±2.06 mg/min-kg; P<0.05 vs healthy controls). In patients responding to antiviral therapy, ABT and GEC returned to normal values after 3 mo (0.82±0.14% dose-kg/mmol CO2 and 7.84±1.71 mg/min-kg.), showing a statistically significant change compared to the initial values (P<0.05). Non-responders still showed reduced parameters of microsomal and cytosolic liver function after the therapy interval (ABT: 0.68±0.19% dose-kg/mmol CO2; GEC: 5.89±1.64 mg/min-kg). Paralleling transaminase levels, ABT and GEC showed a high correlation with HCV-RNA levels with r = 0.974 and r = 0.99, respectively.
In contrast to these metabolic liver function tests, no changes were observed in QTLF investigating parameters of liver perfusion. While SCl (Figure 1C) and ICG (Figure 1D) were lower in patients with hepatitis C compared to healthy controls (not significant, P>0.05), neither responders nor non-responders to antiviral therapies improved during the 3 mo treatment period. SCl was 12.86±2.58 mL/min-kg in healthy controls, 10.8±2.19 mL/min-kg in HCV patients before treatment and 11.3±2.13 and 10.72±2.37 mL/min-kg in responders and non-responders to antiviral therapy. ICG was determined as 0.21±0.11 L/min in controls, 0.17±0.07 L/min before treatment, 0.20±0.07 L/min in responders and 0.18±0.09 L/min in non-responding patients, respectively. Values for ICG and SCl did not correlate with HCV-RNA or transaminases. Although both parameters show slight differences in responders before and after antiviral therapy, these changes were statistically not significant.
Overall, antiviral therapy ameliorated QTLF of microsomal (ABT) and cytosolic (GEC) metabolic capacities, but did not influence parameters of liver perfusion (SCl) and secretion (ICG). Amelioration of ABT and GEC was independent of the applied antiviral regimen in the examined patient groups, but only dependent on overall response to treatment as evidenced by high correlation coefficients with serum transaminase levels.
We have shown previously that the application of a broad panel of QTLF is a useful diagnostic tool to assess prognosis and outcome in different liver diseases[8,13,14]. Although liver damage due to chronic viral hepatitis is considered more predictable than toxic injuries, so far only the application of a single QTLF has been described in small collectives[19,20] and we have shown recently that untreated viral hepatitis influences QTLF in a large cohort[12]. Additionally, we have shown previously that QTLF are inversely correlated with Child-Pugh grades in cirrhotic patients[8]. As there is no information available on the impact of antiviral therapies using INF, INF/RBV or PEG-INF/RBV, we applied a broad panel of QTLF to 50 consecutive patients with chronic HCV infection, 3 mo after beginning the antiviral therapy. At this time point, therapy response is usually re-evaluated to distinguish responders from non-responders to antiviral treatment. HCV has been shown to lead to parenchymal inflammation and to decrease microsomal as well as cytosolic hepatic enzyme capacities, which was independent of the etiology of hepatitis[12].
Compared to healthy controls, we found a significant reduction in metabolic liver function tests (ABT, GEC) in all HCV patients before the initiation of either antiviral therapy. ABT is mainly used to evaluate the prognosis of alcohol-induced hepatitis, paracetamol poisoning or cirrhosis[12,21-23]. ABT can be influenced by pharmacologic induction (e.g., barbiturates, phenytoine) or inhibition (e.g., contraceptives) of microsomal enzymes[14].
GEC was proposed as a prognostic parameter in chronic hepatitis as well as acute liver failure and has been demonstrated to be already reduced in patients with mild fibrosis or acute liver dysfunction[11,22,24].
In HCV patients, reduction of ABT and GEC can be explained by decreased enzymatic capacities caused by active viral replication and expression of viral proteins. Additionally, the total number of vital hepatocytes is decreased in chronic HCV due to ongoing parenchymal inflammation, tissue remodeling and scarring by advancing fibrosis/cirrhosis due to immunologic responses[25]. As responders to antiviral treatment, HCV-RNA was not detectable after 3 mo, independent of the therapy regimen and both metabolic parameters increased to the levels of healthy controls indicating that viral replication strongly influences both microsomal and cytosolic enzymes. HCV is a rapidly replicating virus whose genome is translated into a large polyprotein precursor which undergoes extensive post-translational modifications[26] by using different cellular enzyme systems which might explain lower metabolic QTLF values in HCV patients.
In contrast to the metabolic parameters, QTLF investigating hepatic perfusion (SCl and ICG) were not significantly reduced in the 50 patients with chronic HCV infection and remained unaffected by any response to antiviral therapies. This corroborates previous findings, where hepatic perfusion remained in the normal range even in patients with early fibrotic lesions and irrespective of inflammatory grade of size of the lesions[12]. ICG is considered to be insensitive to pharmacological influences, because its metabolism solely depends on hepatic transport and biliary secretion. Here, we found that 3 mo of different antiviral therapies did not influence ICG, as was shown previously for long-term treatment with beta-blockers, nitrates or antithyroid medications[9,27].
In summary, our results suggest that QTLF monitoring metabolic liver functions (ABT, GEC) may be a useful tool to discriminate responders from non-responders to antiviral therapy regimens in chronic HCV infection after a 3-mo treatment period. While liver perfusion is not altered by either HCV infection or antiviral treatment, ABT and GEC may help to optimize economic and medical treatment strategies in chronic viral hepatitis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 398] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 2. | Everson GT. Treatment of chronic hepatitis C in patients with decompensated cirrhosis. Rev Gastroenterol Disord. 2004;4 Suppl 1:S31-S38. [PubMed] |
| 3. | McHutchison JG, Poynard T, Esteban-Mur R, Davis GL, Goodman ZD, Harvey J, Ling MH, Garaud JJ, Albrecht JK, Patel K. Hepatic HCV RNA before and after treatment with interferon alone or combined with ribavirin. Hepatology. 2002;35:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, Sarrazin C, Harvey J, Brass C, Albrecht J. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 254] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 6. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 7. | Shiffman ML. Management of patients with chronic hepatitis C virus infection and previous nonresponse. Rev Gastroenterol Disord. 2004;4 Suppl 1:S22-S30. [PubMed] |
| 8. | Herold C, Heinz R, Radespiel-Tröger M, Schneider HT, Schuppan D, Hahn EG. Quantitative testing of liver function in patients with cirrhosis due to chronic hepatitis C to assess disease severity. Liver. 2001;21:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Merkel C, Gatta A, Zoli M, Bolognesi M, Angeli P, Iervese T, Marchesini G, Ruol A. Prognostic value of galactose elimination capacity, aminopyrine breath test, and ICG clearance in patients with cirrhosis. Comparison with the Pugh score. Dig Dis Sci. 1991;36:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Merkel C, Marchesini G, Fabbri A, Bianco S, Bianchi G, Enzo E, Sacerdoti D, Zoli M, Gatta A. The course of galactose elimination capacity in patients with alcoholic cirrhosis: possible use as a surrogate marker for death. Hepatology. 1996;24:820-823. [PubMed] [DOI] [Full Text] |
| 11. | Reichen J, Widmer T, Cotting J. Accurate prediction of death by serial determination of galactose elimination capacity in primary biliary cirrhosis: a comparison with the Mayo model. Hepatology. 1991;14:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Herold C, Heinz R, Niedobitek G, Schneider T, Hahn EG, Schuppan D. Quantitative testing of liver function in relation to fibrosis in patients with chronic hepatitis B and C. Liver. 2001;21:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Herold C, Regn S, Ganslmayer M, Ocker M, Hahn EG, Schuppan D. Can quantitative tests of liver function discriminate between different etiologies of liver cirrhosis? Dig Dis Sci. 2002;47:2669-2673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Herold C, Ganslmayer M, Ocker M, Zopf S, Gailer B, Hahn EG, Schuppan D. Inducibility of microsomal liver function may differentiate cirrhotic patients with maintained compared with severely compromised liver reserve. J Gastroenterol Hepatol. 2003;18:445-449. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Hepner GW, Vesell ES. Quantitative assessment of hepatic function by breath analysis after oral administration of (14C)aminopyrine. Ann Intern Med. 1975;83:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 150] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Tygstrup N, Andersen PK, Thomsen BL. Prognostic evaluation in alcoholic cirrhosis. Acta Med Scand Suppl. 1985;703:149-156. [PubMed] |
| 17. | Zeeh J, Lange H, Bosch J, Pohl S, Loesgen H, Eggers R, Navasa M, Chesta J, Bircher J. Steady-state extrarenal sorbitol clearance as a measure of hepatic plasma flow. Gastroenterology. 1988;95:749-759. [PubMed] |
| 18. | Leevy CM, Mendenhall CL, Lesko We, Howard MM. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962;41:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 284] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Horsmans Y, Brenard R, Geubel AP. Short report: interferon-alpha decreases 14C-aminopyrine breath test values in patients with chronic hepatitis C. Aliment Pharmacol Ther. 1994;8:353-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Reichen J, Bianchi L, Buhler H, Dolivo N, Gonvers JJ, Lavanchy D, Male PJ, Renner EL, Solioz M, Schmid M. Fixed versus titrated interferon-alpha 2B in chronic hepatitis C. A randomized controlled multicenter trial. The Swiss Association for the Study of the Liver. J Hepatol. 1996;25:275-282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Saunders JB, Lewis KO, Paton A. Early diagnosis of alcoholic cirrhosis by the aminopyrine breath test. Gastroenterology. 1980;79:112-114. [PubMed] |
| 22. | Saunders JB, Wright N, Lewis KO. Predicting outcome of paracetamol poisoning by use 14C-aminopyrine breath test. Br Med J. 1980;280:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Giannini E, Fasoli A, Chiarbonello B, Malfatti F, Romagnoli P, Botta F, Testa E, Polegato S, Fumagalli A, Testa R. 13C-aminopyrine breath test to evaluate severity of disease in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2002;16:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Ranek L, Andreasen PB, Tygstrup N. Galactose elimination capacity as a prognostic index in patients with fulminant liver failure. Gut. 1976;17:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 26. | Moradpour D, Brass V, Gosert R, Wölk B, Blum HE. Hepatitis C: molecular virology and antiviral targets. Trends Mol Med. 2002;8:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Andersen V, Sonne J, Court-Payen M, Sletting S, Prip A, Molholm-Hansen J. Liver volume, portal vein flow, and clearance of indocyanine green and antipyrine in hyperthyroidism before and after antithyroid treatment. Scand J Gastroenterol. 1999;34:618-622. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |