Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5498
Revised: December 20, 2004
Accepted: December 21, 2004
Published online: September 21, 2005
AIM: To clarify the types, regional distributions and distribution densities as well as morphological features of gastrointestinal (GI) endocrine cells in various parts of the gastrointestinal track (GIT) of four reptiles, Gekko japonicus, Eumeces chinensis, Sphenomorphus indicus and Eumeces elegans.
METHODS: Paraffin-embedded sections (5 μm) of seven parts (cardia, fundus, pylorus, duodenum, jejunum, ileum, rectum) of GIT dissected from the four reptiles were prepared. GI endocrine cells were revealed by using immunohistochemical techniques of streptavidin-peroxidase (S-P) method. Seven types of antisera against 5-hydroxy-tryptamine (5-HT), somatostatin (SS), gastrin (GAS), glucagon (GLU), substance P (SP), insulin and pancreatic polypeptide were identified and then GI endocrine cells were photomicrographed and counted.
RESULTS: The GI endocrine system of four reptiles was a complex structure containing many endocrine cell types similar in morphology to those found in higher vertebrates. Five types of GI endocrine cells, namely 5-HT, SS, GAS, SP and GLU immunoreactive (IR) cells were identified in the GIT of G. japonicus, E. chinensis and S. indicus; while in the GIT of E. elegans only the former three types of endocrine cells were observed. No PP- and INS- IR cells were found in all four reptiles. 5-HT-IR cells, which were most commonly found in the pylorus or duodenum, distributed throughout the whole GIT of four reptiles. However, their distribution patterns varied from each other. SS-IR cells, which were mainly found in the stomach especially in the pylorus and/or fundus, were demonstrated in the whole GIT of E. chinensis, only showed restricted distribution in the other three species. GAS-IR cells, with a much restricted distribution, were mainly demonstrated in the pylorus and/or the proximal small intestine of four reptiles. GLU-IR cells exhibited a limited and species-dependent variant distribution in the GIT of four reptiles. SP-IR cells were found throughout the GIT except for jejunum in E. elegans and showed a restricted distribution in the GIT of G. japonicus and S. indicus. In the GIT of four reptiles the region with the highest degree of cell type heterogeneity was pylorus and most types of GI endocrine cells along the GIT showed the peak density in pylorus as well.
CONCLUSION: Some common and unique features of the distribution and morphology of different types of GI endocrine cells are found in four reptiles. This common trait may reflect the similarity in digestive physiology of various vertebrates.
- Citation: Huang XG, Wu XB. Immunohistochemical study on gastrointestinal endocrine cells of four reptiles. World J Gastroenterol 2005; 11(35): 5498-5505
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5498.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5498
Today more than 30 GI hormone genes and a multitude of GI hormones have been recognized, thus making the GIT the largest endocrine organ in the body[1]. GI hormones as regulatory peptides appear to be the major components of body integration and have important regulatory actions on physiological function of the GIT[2-6]. In recent years, the field of GI hormones expanding at a dazzling speed has become an important domain in gastroenterology. Some GI dysfunctions are related to GI hormones[7]. Some GI hormones have synergistic expression in gastric carcinoma and take part in the occurrence of gastric carcinoma[8,9]. Furthermore, some GI hormones in combination with their peptide analogs are helpful in developing new diagnostic and therapeutic strategies[10]. The physiological role of hormonal messengers, peptide receptors and their potential involvement in disease are under investigation.
GI endocrine cells, dispersed in epithelia and gastric gland of the GIT, are the anatomical units responsible for the production of GI hormones. Since pathological changes of GIT also correlate with the changes of GI endocrine cells[11-15], the investigation of GI endocrine cells has been considered as an important part of GI hormone studies and can provide baseline data for basic research in gastroenterology.
It is generally accepted that GI endocrine cells are remarkably different in regional distribution, relative frequency, cell types, and each regional part of the GIT. Many studies have elucidated the regional distribution and relative frequency of different endocrine cells in the GIT of other vertebrates, such as fishes, amphibians[16,17], especially mammals[18-22]. Some studies on regional distribution and relative frequency or distribution density of GI endocrine cells of reptilian species have also been carried out[23-28], because they are centrally placed on the vertebrate phylogenetic tree.
The present study was conducted to clarify the types, regional distribution, distribution densities and morphology of GI endocrine cells in the GIT of G. japonicus, E. chinensis, S. indicus and E. elegans by specific immunohistochemical methods, and to provide baseline data not only for further morphological studies but also for physiological and pathological research on the digestive system of reptilians.
G. japonicus (n = 5, body length: 13014 mm) in the campus of Anhui Normal University, E. chinensis (n = 3, body length: 18020 mm) in the campus of the 9th Middle School in Wuhu City, S. indicus (n = 5, body length: 19018 mm) and E. elegans (n = 5, body length: 15010 mm) in Langya Mountain were collected in summer and autumn. The animals were lightly anesthetized with ether and decapitated with their cardia, fundus, pylorus, duodenum, jejunum, ileum and rectum dissected and fixed in Bouin’s fluid for 24 h. After being dehydrated through an ethanol-xylene series the specimens were embedded in paraffin. Sections were cut at 5 μm thickness and mounted on gelatin-coated slides.
The details of the seven antisera and main reagents used in this study are listed in Table 1.
| Antisera and reagent | Donor | Code number | Working dilution | Source |
| Human 5-HT | Rabbit | ZA-0231 | 1:100 | ZYMED Lab. Inc., USA |
| Human GAS | Rabbit | ZA-0115 | 1:50 | Same as above |
| Human SS | Rabbit | ZA-0232 | 1:50 | Same as above |
| Human PP | Rabbit | ZA-0211 | 1:50 | Same as above |
| Human SP | Rabbit | ZA-0235 | Working solution | Same as above |
| Human GLU | Rabbit | ZA-0119 | 1:100 | Same as above |
| Human INS | Mouse | ZM-0155 | 1:50 | Same as above |
| S-P Kit | SP-9001 | Working solution | Zhongshan Biotech Co. LTD., Beijing, China | |
| S-P Kit | SP-9002 | Working solution | Same as above | |
| DAB | ZLI-9030 | 0.6 g/L | Same as above |
Each representative section was deparaffinized, rehydrated and immunostained with the S-P method. Endogenous peroxidase activity was blocked by incubating the sections with 0.3% H2O2 in methanol for 10 min. The non-specific reaction was blocked with normal goat serum prior to overnight incubation at 4 °C with the primary antiserum (Table 1). After being rinsed in phosphate-buffered saline (PBS; 0.01 mol/L, pH 7.4), the sections were incubated for 15 min at room temperature with biotinylated goat anti-rabbit IgG serum for all antisera, while biotinylated goat anti-mouse IgG serum was used for insulin. They were then washed in PBS and incubated for 15 min with S-P. Peroxidase reaction was carried out in a solution of DAB containing 0.01% H2O2 in Tris-HCl buffer (0.05 mol/L, pH 7.6). After the being lightly counterstained with Mayer’s hematoxylin, the sections were dehydrated and cover-slipped.
To investigate the specificity of the reactions, negative and positive controls were used. By replacement of the primary antisera with normal goat serum and PBS, negative control was set. A positive control was also established using tissue sections from the GIT of tortoise containing the hormones being studied. The positive reaction cells were colored brown.
All animals were used for each of the four reptiles studied, seven specimens of each reptile were observed and photomicrographed under the Olympus BX-51 photom-icroscope. The dark-brown positive reaction cells on the sections were counted under 1040 times field. The average number of positive reaction cells from the 10 fields selected randomly in each specimen was the number of IR cells in each reptile. Then the average number of all the reptiles was again quantified as distribution density of IR cells.
Data were expressed as mean±SD and variance analysis was performed using SPSS 11.0 software. One-way analysis of variance was used for multiple comparisons, and Duncan’s test was used for intra-group comparisons. P<0.05 was considered statistically significant.
Five types of endocrine cells namely 5-HT-, SS-, GAS-, SP- and GLU-IR cells were identified in the GIT of G. japonicus, E. chinensis and S. indicus. However, in the GIT of E. elegans only 5-HT-, SS-, GAS-IR cells were found. PP- and INS- IR cells were not detected in the GIT of the four reptiles. The regional distribution and distribution density of GI endocrine cells in the GIT of four reptiles are listed in Tables 2-5. No positive reactions were detected in the negative control sections.
| 5-HT | SS | GAS | GLU | SP | PP | INS | |
| Cardia | 4.8±0.8 | 5.5±1.9 | 0 | 4.3±1.2 | 3.6±1.1 | 0 | 0 |
| Fundus | 5.6±1.4 | 6.0±1.2 | 0 | 11.7±0.7 | 14.6±1.8 | 0 | 0 |
| Pylorus | 4.1±1.5 | 9.6±1.0 | 0 | 16.3±2.2 | 11.2±2.2 | 0 | 0 |
| Duodenum | 8.6±2.2 | 1.0±0.5 | 3.8±0.8 | 0 | 0 | 0 | 0 |
| Jejunum | 7.0±2.9 | 0 | 1.4±0.5 | 0 | 0 | 0 | 0 |
| Ileum | 7.8±1.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rectum | 7.0±1.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5-HT | SS | GAS | GLU | SP | PP | INS | |
| Cardia | 1.6±1.1 | 1.1±1.2 | 0 | 0 | 0 | 0 | 0 |
| Fundus | 4.2±1.0 | 2.3±0.7 | 0 | 0 | 0 | 0 | 0 |
| Pylorus | 9.1±1.7 | 4.6±1.9 | 21.4±11.6 | 0 | 0 | 0 | 0 |
| Duodenum | 3.1±0.9 | 0 | 1.3±1.1 | 0 | 0 | 0 | 0 |
| Jejunum | 1.4±1.4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ileum | 3.4±1.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rectum | 5.2±2.0 | 0 | 0 | 0 | 0 | 0 | 0 |
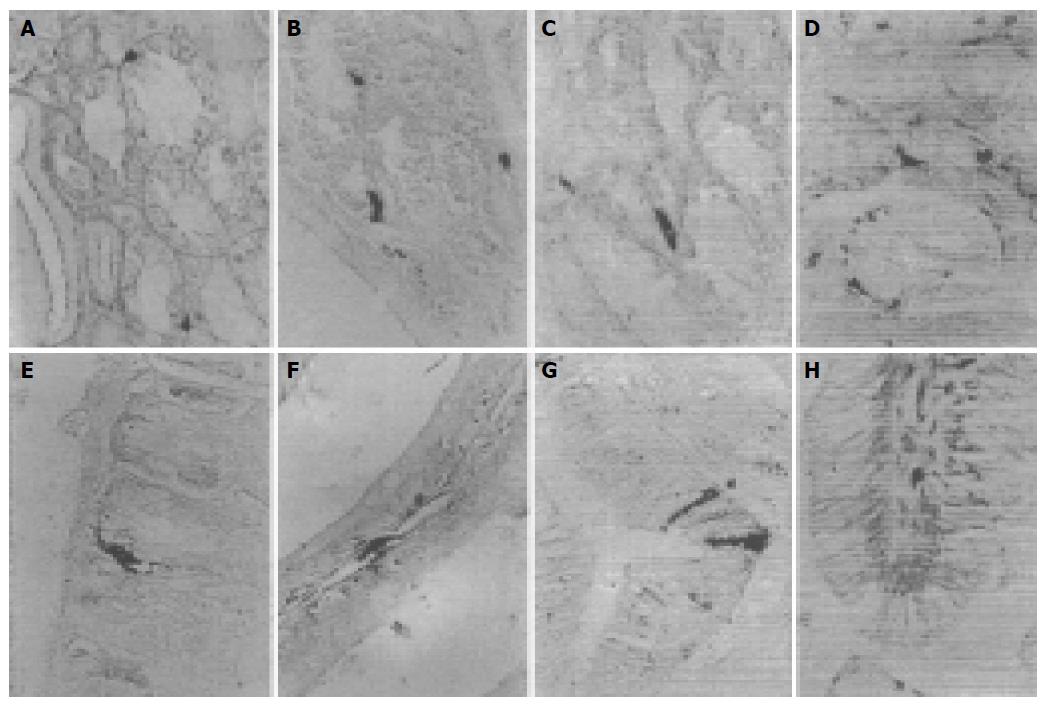
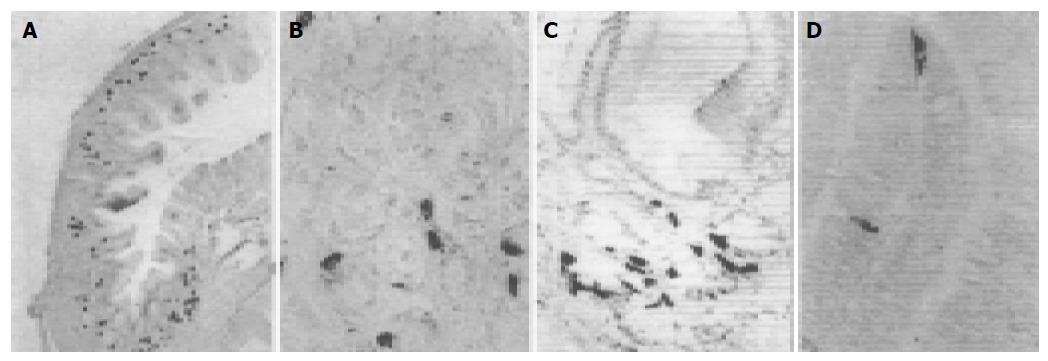
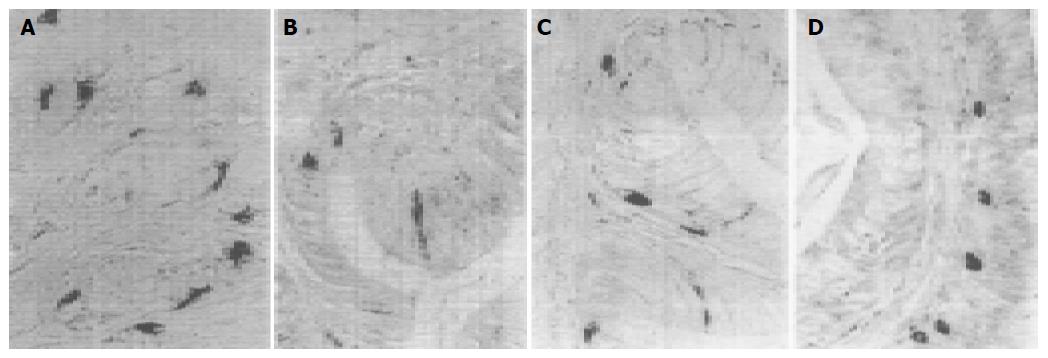
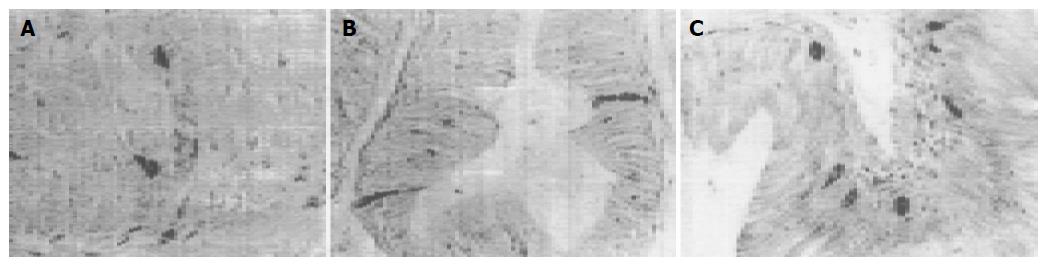
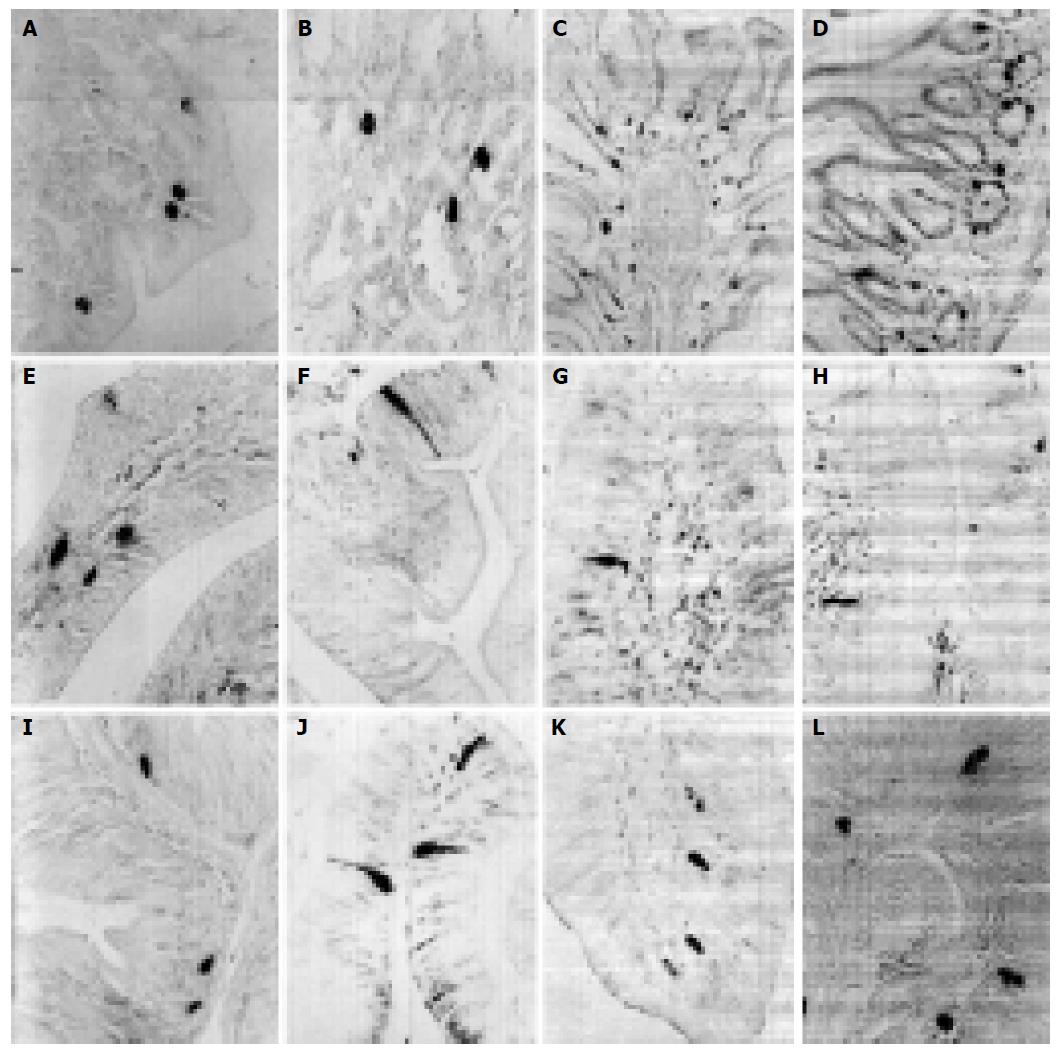
5-HT-IR cells detected throughout the whole GIT of four reptiles were the most predominant GI endocrine cells. These cells showed the highest density in pylorus of four reptiles except for G. japonicus in which the most abundant cells were observed in the duodenum. However, the distribution pattern of 5-HT-IR cells in the GIT varied considerably in four reptiles (Figure 1). SS-IR cells were demonstrated in the whole GIT of E. chinensis, but only showed restricted distribution in the other three. They were found in stomach and duodenum of G. japonicus and only in stomach of S. indicus and E. elegans. SS-IR cells were most predominant in pylorus of four reptiles except for G. japonicus in which most SS-IR cells were detected in fundus (Figure 2). GAS-IR cells showed limited distribution in the GIT of four reptiles and were confined to pylorus of E. chinensis, pylorus and duodenum of S. indicus and E. elegans, duodenum and jejunum of G. japonicus, respectively. In the pylorus of E. elegans, GAS-IR cells showed the highest density (Figure 3). GLU-IR cells were detected in the whole stomach of G. japonicus, in ileum and rectum of S. indicus, in ileum and rectum, and rarely in fundus of E. chinensis (Figure 4). SP-IR cells distributed throughout the GIT except for jejunum of E. elegans, and were found in stomach of G. japonicus and in rectum of S. indicus respectively (Figure 5). In the GIT of four reptiles, the region with the highest degree of cell type heterogeneity was the pylorus and most types of GI endocrine cells in the GIT showed the peak density in pylorus as well.
The GI endocrine system of four reptiles contained many endocrine cell types similar in morphology to those found in higher vertebrates. The endocrine cells showed the characteristic form of both open and close types. They appeared as close-type cells as they did not possess lamina contact with their apical cytoplasmic processes and as open-type cells with apical cytoplasmic processes that reached the glandular or intestinal lumen. The GI endocrine cells were round, oval, triangular, spindle-, shuttle- or flask-like in shape. They were mostly found in gastric glands and intestinal epithelium, and occasionally in gastric epithelia. In gastric glands, the GI endocrine cells were located between the glandular cells and the basement membrane at the basal portion of the glands. In the intestinal parts, most of these cells were situated in the basal portion of the epithelia.
The present study demonstrated that the GI endocrine system of four reptiles is a complex structure containing many endocrine cell types similar to those found in mammals except for PP-IR cells[19-22]. 5-HT-, SS-, GAS-, GLU-, SP-IR cells were identified in the GIT of four reptiles, except for E. elegans, in which only the former three types were found.
5-HT, a monoamine, which has a strong effect on regulation of digestive functions, is widely distributed in gastric epithelial endocrine cells, 5-HT-IR cells[4,23]. El-Salhy et al[23] reported that 5-HT-IR cells are detected throughout the GIT of all species of vertebrates, suggesting that they have been established in the GIT at an early stage of vertebrate evolution. By using specific antiserum against 5-HT, these cells were detected throughout the GIT of four reptiles in the present study, further approving that 5-HT-IR cells have a wider distribution than other types of GI endocrine cells in GIT of vertebrates. Similarly to Ku et al[29] and Arena et al[30] 5-HT-IR cells are most predominant in pylorus of E. chinensis, S. indicus and E. elegans. In other studies[24,25,28,31], the highest frequency of 5-HT-IR cells is in the duodenum and similar results are found in G. japonicus, suggesting that pylorus and/or duodenum are important parts in GIT for digestion and a great deal of 5-HT is needed to facilitate the digestive process. As for the distribution pattern (regional distribution and relative frequency or distribution density in GIT) of 5-HT-IR cells in the GIT of four reptiles, considerable variation was observed, and no one was similar to each other, suggesting that the distribution pattern of 5-HT-IR cells may vary from different species. In the present study, some 5-HT-IR cells in the stomach of four reptiles exhibited narrow apical cytoplasmic processes reaching adjacent cells or the gastric gland lumen, indicating that these cells can probably secrete 5-HT by paracrine pathway. In the intestines of four reptiles, 5-HT-IR cells with cytoplasmic processes reaching intestinal lumen were also observed, suggesting that 5-HT may be secreted by exocrine pathway to intestinal lumen.
In most mammals as well as some reptiles, SS-IR cells have a wide distribution in the GIT, except for the large intestine[29-33]. However, species-dependent variations in the distribution patterns of these IR cells have also been reported[24-28]. In the present study, SS-IR cells were demonstrated in the whole GIT of E. chinensis, which are in agreement with those reported in Trimeresurus stejnegeri, Trionyx sinensis, Alligator mississippiensis and Caiman latirostris[24-27]. In the other three reptiles, SS-IR cells showed restricted distribution. In the GIT of G. japonicus, SS-IR cells were demonstrated in stomach and duodenum, as in Alligator sinensis[28]. In the GIT of S. indicus and E. elegans, SS-IR cells are only restricted to the stomach, which differ from those of previous reports on other reptiles[29-33]. These studies suggest that the distribution pattern of SS-IR cells varies greatly in reptiles. On the other hand, common distribution features of SS-IR cells also exist. Studies show that in the GIT of other vertebrates especially mammals, SS-IR cells are found mainly in stomach[20,22], and this common place is also found in most reptiles[27-32]. In the present study SS-IR cells were most predominant in pylorus of E. chinensis, S. indicus and E. elegans. This finding is in line with those of most vertebrates[29,31]. Somewhat different from the above results, SS-IR cells were most abundant in fundus rather than in pylorus of G. japonicus, and this difference may be due to the different species. The results found in G. japonicus have also been reported in some mammals[18,19,22]. Larsson et al[5] reported that in the stomach, SS-IR cells have cytoplasmic processes terminated on GAS-producing cells, and SS is secreted through cytoplasmic processes by paracrine pathway, inhibiting the secretion of GAS. In the present study, both SS- and GAS-IR cells showed the highest density in pylorus of four reptiles except for G. japonicus, suggesting that some relationships may exist between them. Some SS-IR cells with cytoplasmic processes extending to adjacent cells were also found, suggesting that SS-GAS paracrine regulation may exist in reptiles as in mammals.
In some reptiles, GAS-IR cells are observed in pylorus and whole small intestine of GIT[25,30-33], while in the present study, GAS-IR cells showed restricted distribution in the GIT of four reptiles and the results are similar to those reported in most mammals[18-22]. GAS-IR cells in pylorus and duodenum of S. indicus and E. elegans are reported in Bubalus bubalis and Caiman latirostris[20,27]. In the GIT of E. chinensis, GAS-IR cells are confined to the pylorus, corresponding to those found in C57BL/6 mice and SKH-1 hairless mice[18,19]. Interestingly, GAS-IR cells were not detected in the pylorus but in the duodenum and jejunum of G. japonicus, which differ from those of other three reptiles in which GAS-IR cells were numerous in the pylorus. However, this distribution is consistent with that in Rana japonica japonica[17]. In addition, GAS-IR cells are observed in the intestines of Alligator sinensis but not in the pylorus[28]. GAS secreted by GAS-IR cells, regulate gastric acid secretion[6]. In E. chinensis, S. indicus and E. elegans, like most vertebrates[30-32], the highest density of GAS-IR cells is found in the pylorus, and this distribution may be important for them to fulfill the regulation of digestive function. However, GAS-IR cells were detected in small intestine in G. japonicus but not in the pylorus. Gut hormones have been demonstrated to release into tissue spaces in a paracrine way, they can also release into the gut lumen in a partial exocrine manner or into the blood in an endocrine fashion[3]. In the present study, GAS-IR cells in the intestines of G. japonicus were often found with cytoplasmic processes either reaching intestinal lumen or extending to lamina propria, suggesting an exocrine or endocrine secretion of these cell types. Maybe in this way, GAS-IR cells in the intestine can regulate gastric acid secretion. Studies are required to elucidate the functional meaning of these findings.
GLU is synthesized in A cells of the pancreas and regulates serum glucose levels. GLU-IR cells have been demonstrated in various mammals and it is considered that the distribution pattern of these cells in GIT of mammals show species-dependent variation[18-22]. In the present study no GLU-IR cells were found in GIT of E. elegans and the findings are similar to those in Trionyx sinensis, Alligator sinensis and Egernia kingii and remarkably different from those in Caiman latirostris in which GLU-IR cells were detected throughout the GIT except for the cloaca[25,27,28,30]. In GIT of G. japonicus, GLU-IR cells are only found in the stomach, corresponding well with those in Alligator mississippiensis[26]. In GIT of E. chinensis and S. indicus GLU-IR cells are mainly localized in the ileum and rectum. The above studies suggest that the regional distribution of GLU-IR cells in the GIT varies in reptilians.
Up to date, reports on SP-IR cells in the GIT of reptiles are scarce[24,28,31,33,34]. The present study showed that SP-IR cells exhibited variant distribution patterns in GIT of four reptiles. In E. chinensis SP-IR cells were found throughout the GIT except for jejunum. Similar to Lacerta lepida[31], SP-IR cells are confined to the stomach in GIT of G. japonicus. The GIT of E. elegans is devoid of SP-IR cells, which is consistent with that in Trimeresurus stejnegeri, Alligator sinensis, Testudo graeca and Mauremys caspica[24,28,31]. In S. indicus SP-IR cells are only found in the rectum, which are quite different from those of previous studies[33,34].
In this study, the region with the highest degree of cell type heterogeneity was the pylorus. The distribution of most endocrine cell types in the GIT showed the peak density in the pylorus as well. The heterogeneity and concentration of endocrine cells in the pylorus may be attributed to the role of the cells in the feedback control of the function of the segment. In G. japonicus and E. elegans the spectrum of cell types in the intestine gradually narrowed. While in E. chinensis and S. indicus the spectrum gradually narrowed from duodenum to jejunum and then widened and got its minimum in jejunum and maximum in rectum.
In conclusion, some common features of the distribution and morphology of different types of GI endocrine cells are found in GIT of four reptiles. This common trait may reflect the similarity in digestive physiology of various vertebrates. Further physiological studies are required to elucidate the ways in which the distribution of GI endocrine cells may be related to the regulatory characteristics of the GIT of reptiles.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Ahlman H. The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12 Suppl 2:S63-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Solcia E, Rindi G, Buffa R, Fiocca R, Capella C. Gastric endocrine cells: types, function and growth. Regul Pept. 2000;93:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Solcia E, Capella C, Vassallo G, Buffa R. Endocrine cells of the gastric mucosa. Int Rev Cytol. 1975;42:223-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 213] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Larsson LI, Goltermann N, de Magistris L, Rehfeld JF, Schwartz TW. Somatostatin cell processes as pathways for paracrine secretion. Science. 1979;205:1393-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 345] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Larsson LI. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc Res Tech. 2000;48:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Milutinovic AS, Todorovic V, Milosavljevic T, Micev M, Spuran M, Drndarevic N. Somatostatin and D cells in patients with gastritis in the course of Helicobacter pylori eradication: a six-month, follow-up study. Eur J Gastroenterol Hepatol. 2003;15:755-766. [PubMed] |
| 8. | Henwood M, Clarke PA, Smith AM, Watson SA. Expression of gastrin in developing gastric adenocarcinoma. Br J Surg. 2001;88:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Watson SA, Morris TM, McWilliams DF, Harris J, Evans S, Smith A, Clarke PA. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br J Cancer. 2002;87:567-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Grimberg A. Somatostatin and cancer: applying endocrinology to oncology. Cancer Biol Ther. 2004;3:731-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | El-Salhy M, Sitohy B. Abnormal gastrointestinal endocrine cells in patients with diabetes type 1: relationship to gastric emptying and myoelectrical activity. Scand J Gastroenterol. 2001;36:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Tzaneva MA. Endocrine cells in gastric carcinoma and adjacent mucosa. An immunohistochemical and ultrastructural study. Histochem J. 2002;34:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Tzaneva M. Effects of duodenogastric reflux on gastrin cells, somatostatin cells and serotonin cells in human antral gastric mucosa. Pathol Res Pract. 2004;200:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Zhao R, Baig MK, Wexner SD, Chen W, Singh JJ, Nogueras JJ, Woodhouse S. Enterochromaffin and serotonin cells are abnormal for patients with colonic inertia. Dis Colon Rectum. 2000;43:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Tzaneva M. Light and electron microscopic immunohistochemical investigation on G and D cells in antral mucosa in Helicobacter pylori-related gastritis. Exp Toxicol Pathol. 2001;52:523-528. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Zhang SZ, Huang XG, Wu XB. Immunohistochemical studies on endocrine cells in the digestive tract of Paa spinosa. Dongwu Xuebao. 2003;49:858-864. |
| 17. | Huang XG, Wu HL, Wu XB, Zhang SZ. Immunohistochemical studies on endocrine cells in the gastrointestinal tract of Rana japonica japonica. Dongwuxue Zazhi. 2004;39:19-25. |
| 18. | Ku SK, Lee JH, Lee HS, Park KD. The regional distribution and relative frequency of gastrointestinal endocrine cells in SHK-1 hairless mice: an immunohistochemical study. Anat Histol Embryol. 2002;31:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Ku SK, Lee HS, Lee JH. An immunohistochemical study of the gastrointestinal endocrine cells in the C57BL/6 mice. Anat Histol Embryol. 2003;32:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Baltazar ET, Kitamura N, Hondo E, Yamada J, Maala CP, Simborio LT. Immunohistochemical study of endocrine cells in the gastrointestinal tract of the Philippine carabao (Bubalus bubalis). Anat Histol Embryol. 1998;27:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Eerdunchaolu DV, Takehana K, Kobayashi A, Yamada J, Ueda H, Baiyin GF, Abe M. Immunohistochemical study of the distribution of endocrine cells in the gastrointestinal tract of the camel (Camelus bactrianus). Eur J Morphol. 2001;39:57-63. [PubMed] [DOI] [Full Text] |
| 22. | Agungpriyono S, Macdonald AA, Leus KY, Kitamura N, Adnyane IK, Goodall GP, Hondo E, Yamada J. Immunohistochemical study on the distribution of endocrine cells in the gastrointestinal tract of the babirusa, Babyrousa babyrussa (Suidae). Anat Histol Embryol. 2000;29:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | El-salhy M, Wilander E, Lundqvist M. Comparative studies of serotonin-like immuno- reactive cells in the digestive tract of vertebrates. Biomedical Res. 1985;6:371-375. |
| 24. | Zhang ZQ, Zhang SZ, Wu XB. Immunohistochemical localization of the endocrine cells in the digestive tract of Trimeresurus stejnegeri. Dongwu Xuebao. 2001;47:666-671. |
| 25. | Lin SG, Huang ZJ, Wang SK. Localization and identification of endocrine cells in the Gastro-Entero-Pancreatic system of Trionyx sinensis. Shuichan Xuebao. 2001;25:328-333. |
| 26. | Buchan AM, Lance V, Polak JM. Regulatory peptides in the gastrointestinal tract of Alligator mississipiensis. An immunocytochemical study. Cell Tissue Res. 1983;231:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Yamada J, Campos VJ, Kitamura N, Pacheco AC, Yamashita T, Yanaihara N. An immunohistochemical study of the endocrine cells in the gastrointestinal mucosa of the Caiman latirostris. Arch Histol Jpn. 1987;50:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Wu XB, Zhang SZ, Chen BH, Deng ZP, Zhou ZX, Wang CL, Nie JS, Xie WS. Immunohistochemical studies on endocrine cells in the digestive tract of Alligator sinensis. Dongwu Xuebao. 1999;45:155-161. |
| 29. | Ku SK, Lee HS, Lee JH, Park KD. An immunohistochemical study on the endocrine cells in the alimentary tract of the red-eared slider (Trachemys scripta elegans). Anat Histol Embryol. 2001;30:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Arena PC, Richardson KC, Yamada J. An immunohistochemical study of endocrine cells of the alimentary tract of the King's skink (Egernia kingii). J Anat. 1990;170:73-85. [PubMed] |
| 31. | Perez-Tomas R, Ballesta J, Pastor LM, Madrid JF, Polak JM. Comparative immunohistochemical study of the gastroenteropancreatic endocrine system of three reptiles. Gen Comp Endocrinol. 1989;76:171-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | El-Salhy M, Grimelius L. The endocrine cells of the gastrointestinal mucosa of a squamate reptile, the grass lizard (mabuya quinquetaeniata.) A histological and immunohistochemical study. Biomedical Res. 1981;2:639-658. |
| 33. | Morescalchi AM, Gaccioli M, Faraldi G, Tagliafierro G. The gastro-enteric-pancreatic neuroendocrine system in two reptilian species: Chalcides chalcides and Zoonosaurus madascariensis (Sauridae). Eur J Histochem. 1997;41:29-40. [PubMed] |
| 34. | Huang XG, Wu XB, Zhang ZQ, Zhang SZ. Comparative immunohistochemical study of endocrine cells in the gastrointestinal tract of two reptiles. Zhongguo Zuzhi Huaxue Yu Xibao Huaxue Zazhi. 2003;12:433-440. |