Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5492
Revised: December 23, 2004
Accepted: December 26, 2004
Published online: September 21, 2005
AIM: To study the protective effect of non-mitogenic human acidic fibroblast growth factor (FGF) on cardiac oxidative injury in vivo.
METHODS: Ventricular cardiomyocytes were isolated from 1- to 3-d-old neonatal SD mice and cultured in Dulbecco’s minimum essential medium supplemented with 15% fetal bovine serum under an atmosphere of 50 mL/L CO2-95% air at 37 °C, as well as assessed by immunocyto-chemical assay. We constructed the cardiomyocyte injury model by exposure to a certain concentration of H2O2. Cellular viability, superoxide dismutase (SOD) activity, leakage of maleic dialdehyde and anti-apoptosis effect were included to evaluate the cardiac protective effect of non-mitogenic human acidic FGF.
RESULTS: Over 50% of the cardiomyocytes beat spontaneously on the 2nd d of culture and synchronously beat after being cultured for 3 d. Forty-eight hours after plating was completed, the purity of such cultures was 95% myocytes, assessed by an immunocytochemical assay. Cellular viability dramatically decreased with the increasing of the concentration of H2O2. Non-mitogenic human acidic FGF showed significant resistance to the toxic effect of H2O2, significantly increased the cellular viability as well as the activity of SOD, and dramatically decreased the leakage of maleic dialdehyde as well as the cellular apoptosis rate.
CONCLUSION: Hydrogen peroxide shows strong cytotoxicity to the cultured cardiac myocytes, and non-mitogenic human acidic FGF shows strong cardio-protective effect when exposed to a certain concentration of H2O2.
- Citation: Lin ZF, Li XK, Lin Y, Wu F, Liang LM, Fu XB. Protective effects of non-mitogenic human acidic fibroblast growth factor on hydrogen peroxide-induced damage to cardiomyocytes in vitro. World J Gastroenterol 2005; 11(35): 5492-5497
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5492.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5492
Fibroblast growth factor (FGF) is a family of at least 21 structurally and functionally relevant polypeptides characterized by a high affinity of heparin[1]. Acidic FGF found mainly in the brain and retina as well as in the cardiac tissue is also a multifunctional protein, which has many effects including mitogenesis and non-mitogenesis effects.
Studies indicate that intracoronary, intrapericardial, or myocardial administration of FGFs in chronically ischemic canine and porcine hearts can stimulate angiogenesis, a process to minimize infarct size and improve cardiac function[2]. At the same time, though there are some promising results in animal model trials, it produces acute negative inotropic effects on adult cardiac myocytes resulting from alterations in intracellular Ca2+ homeostasis[3]. Many people wonder if there would be a potential danger of stimulating the growth of tumor, since NIH-3T3 fibroblasts transfected to express constitutively a secreted form of FGF-1 that becomes tumorigenic[4,5]. FGF-1 behaves as a tumorigenic factor in the NBT-II bladder carcinoma cell model[6].
According to the function and structure of human aFGF, by the method of gene engineering, we removed the part of gene related to mitogenesis, eliminated or reduced the power of mitogenesis, and found it was expressed in Escherichia coli. We isolated the non-mitogenic human fibroblast growth factor (nm-haFGF) protein, and justified them by mAb of human FGF. Our relevant research results showed that, after modifying the structure of gene and protein, modified haFGF reduced its mitogenetic effect, and preserved its other bio-effects including anti-apoptosis effect, cardio-protective effect, etc.[7]. In this paper, we isolated and cultured the primary cardiomyocytes from 1- to 3-d neonatal SD mice, and observed the pharmacological cardio-protective effect in vivo.
Nm-haFGF was recombined in our experiment laboratory. DMEM, fetal bovine serum (FBS) and trypsin were purchased from Hyclone Co. (UT, USA). Cell death detection kit and immunocytochemical assay kit were purchased from Boster Co. (Wuhan, China). 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (St. Louis, MO, USA). Superoxide dismutase (SOD) and maleic dialdehyde (MDA) were purchased from Jiancheng Bioengineering Institute of Nanjing, China.
Sprague-Dawley (SD) rats (1-3 d old) were purchased from the Animal Center of Sun Yat-Sen University.
The procedure of culturing ventricular cardiomyocytes from neonatal mice was established by modifications of the previously described methods[7-10]. One- to three-day-old neonatal mice were euthanized by cervical dislocation. Hearts were removed aseptically from the mice, the ventricles were retained, and kept in Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+ (in g/L: 0.4 KCl, 0.06 KH2PO4, 8.0 NaCl, 0.35 NaHCO3 and 0.06 Na2HPO4·7H2O2, pH 7.2) at 4 °C. The ventricles were washed thrice with HBSS and minced into small fragments and washed twice again during mincing. The cells were dissociated at 37 °C for 8 min in enzyme solution (0.8 g/L trypsin in HBSS without Ca2+ and Mg2+, pH 7.2), the ventricle tissue was churned by the magnetic stirring (100 r/min) during the digestion. The cells released after the first digestion were discarded, whereas the cells after subsequent digestion were added to an equal volume of cold HBSS with Ca2+ and Mg2+ (in g/L: 0.14 CaCl2, 0.4 KCl, 0.06 KH2PO4, 0.047 MgCl2, 0.049 MgSO4, 8.0 NaCl, 0.35 NaHCO3, 0.05 Na2HPO4, and 1.0 D-glucose, pH 7.4) until all cardiac cells were isolated (six times). The resulting mixture was centrifuged for 8 min at 200 r/min, and the cells were resuspended in the FBS-MEM (MEM supplemented with 150 mL/L FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin). To exclude the nonmuscle cells, the isolated cells were first plated in tissue culture dishes at 37 °C for 1.5 h in a water-saturated atmosphere of 50 mL/L CO2-95% air based on the observation that nonmuscle cells attached to the substratum more rapidly. The suspended cells were then collected and plated at a density of 1.0×105 cells/cm2 and incubated under the same conditions as mentioned above.
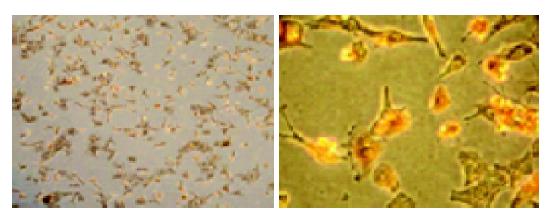
Cardiomyocytes and its purity were monitored by staining with antibody to cardiac α-sarcomeric actin according to the manufacturer’s instructions (Boster). Briefly, myocytes were plated on plastic four-chamber culture slides at 1.0×105 cells/cm2. The cells were fixed with 50% methanol and 50% acetone at 20 °C and then incubated with antigen-specific primary antibody (mouse multiclonal anti-α-sarcomeric-actin antibody diluted at 1:150). After a brief wash, the myocytes were incubated with biotinylated secondary antibody (goat anti-mouse IgM, μ-chain specific). At the addition of an extravidin peroxidase reagent, a stable avidin-biotin complex was formed with the bound biotinylated antibody. The sites of antibody deposition were visualized by the addition of freshly prepared substrate containing H2O2 and chromogen 3-amino-9-ethyl-carbazole. Nuclei were stained with Mayer’s hematoxylin. Myocytes were visualized under a reverse microscope (Olympus).
The cytotoxicity of H2O2 was determined by observing cellular morphology, measuring cellular apoptosis and examining the cell viability. Observing the change of cellular morphology is a traditional method to determine the cellular injury. We observed the change of cellular morphology by reverse microscopy, when cardiomyocytes were exposed to different concentrations of H2O2. Cardiomyocytes were plated on six-well microplates at the density of 1×105 cells/well containing 1 000 μL FBS-DMEM, and cultured in a water-saturated atmosphere of 50 mL/L CO2-95% air box for 48 h. The myocardium was treated with 0.625, 1.25, 2.5, 5, 10 mmol/L H2O2. A photo was taken to record the change of cardiomyocyte morphology after 3 h. The media were removed, the cells were collected and cell genome DNA were extracted, the change of genome DNA was analyzed and the process of cellular apoptosis was evaluated by electrophoresis on 1% agarose gels.
Cell viability was determined by short-term microculture MTT assay. On 96-well microplates, cardiac myocytes were plated at the density of 3×104 cells/well containing 150 μL FBS-DMEM. After being cultured for 48 h, the cells were exposed to different concentrations (0.075, 0.15, 0.3, 0.6 mmol/L) of H2O2 for 3 h, and then the media were replaced by 100 μL of DMEM and 20 μL of MTT solution (5 mg/mL). The cells were incubated for another 4 h. After being incubated for 4 h, the media and MTT solution were removed. The remaining formazan blue crystals were dissolved by MDSO. Absorbance at 570 nm was measured by Thermo Lab Systems (Multiskan MK3).
The cardio-protective effect of nm-haFGF was evaluated on the basis of its anti-oxidic effect of nm-haFGF including measuring the total activity of SOD, monitoring the injury of cells membrane, examining cell viability as well as monitoring cell apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL).
SOD and MDA were measured by respective assay kit (Jiancheng Bioengineering Co.). Cardiomyocytes were plated on 96-well microplates at the density of 3104 cells/well containing 100 μL FBS-DMEM, and cultured in a water-saturated atmosphere of 50 mL/L CO2-95% air box for 48 h. The FBS-DMEM media were replaced by free-serum DMEM containing different concentrations of nm-haFGF (10, 20, 40, 80 ng/mL), and incubated in a water-saturated atmosphere of 50 mL/L CO2-95% air for 24 h, then the cardiomyocytes were treated with 0.15 mmol/L H2O2. The leakage of MDA and the activity of SOD were measured after exposure to H2O2 for 3 h, and the cell viability of cardiomyocytes was measured by MTT.
TUNEL assay was performed. After being treated with nm-haFGF and H2O2, cardiomyocytes were fixed with 4% paraformaldehyde in PBS overnight at 4 °C. The sample was washed with PBS and then permeabilized by 0.2% Triton X-100 in PBS for 5 min at room temperature. After being washed, cells were equilibrated at room temperature for 5-10 min in equilibration buffer (200 mmol/L potassium cacodylate, 0.2 mmol/L dithiothreitol, 0.25 g/L bovine serum albumin, and 2.5 mmol/L cobalt chloride in 25 mmol/L Tris-HCl, pH 6.6) and then incubated in the presence of biotinylated nucleotide mix and terminal deoxynucleotidyl transferase at 37 °C for 1 h in a humidified chamber. The tail reaction was terminated by 2× standard saline citrate (SSC). Then, 0.3% hydrogen peroxide was added to block the endogenous peroxidase. After being washed thrice in PBS, streptavidin HRP was added for 30 min at room temperature, and washed in PBS again. At last, DAB was added to stain the sample, until a suitable background occurred and the positive cells were mounted under a reverse microscope. At least 1 000 cells were counted, and the percentage of TUNEL-positive cells was determined.
Data were expressed as mean±SD. Statistical analysis was performed by Student’s t-test to compare data in different groups. P<0.05 was considered statistically significant.
It was noted that the animal age should not be more than 3 d, because cardiomyocytes obtained from neonatal mice older than 3 d, did not attach to the dishes. After being separated from non-muscle cells by preculturing for 2 h, the cardiomyocytes were replated at a density of 1.0×105 cells/cm2 and incubated under the condition described in Materials and methods. After being cultured for 24 h, almost all the cardiomyocytes attached to and spread on the substratum of the dishes and beat spontaneously. The culture media were changed at this time and then changed every 3 d. Forty-eight hours after culturing was completed, the myocytes were identified by the immunocytochemical assay. The cells isolated and cultured were cardiomyocytes, and the cellular morphological characteristics are shown in Figure 1. The myocyte purity averaged 94±2.25%.
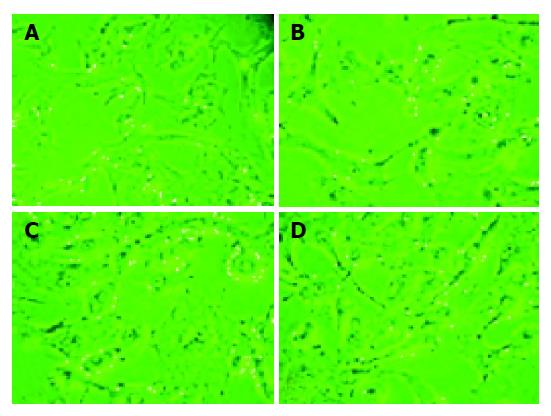
Cardiomyocytes were exposed to different concentrations of H2O2. Morphological alteration, cardiomyocyte apoptosis and cell viability were measured as the cytotoxicity of this oxidant. As shown in Figure 2, a dramatic change of cellular morphology and a concentration-dependent injury effect could be observed. When exposed to 0.6125 mmol/L hydrogen peroxide for 3 h, there were no significant differences in cellular morphology. When the concentration increased, a dramatic change in cellular morphology was found. All the cells lost their characteristics of spontaneous beating, and cellular membrane became shrunk, cellular nuclei were contracted.
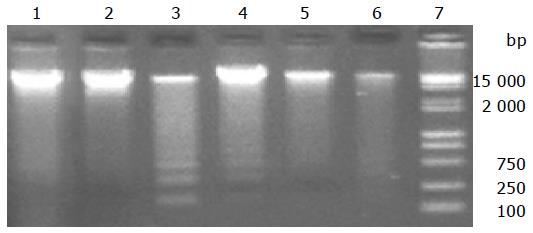
Genomic DNA was measured to indicate alterations in cardiomyocyte apoptosis, when cardiomyocytes were exposed to different concentrations of H2O2. As shown in Figure 3, genomic DNA remained intact after being exposed to 0.625 mmol/L H2O2 for 3 h; when H2O2 concentration was changed to 1.25 mmol/L, genomic DNA was divided into some small fragments; when H2O2 concentration increased to 5 and 10 mmol/L, all genomic DNAs were divided. There were electrophoresis stains, suggesting that apoptosis of cardiomyocytes occurred when they were exposed to H2O2.
MTT assay was used to monitor the cellular viability. There were significant differences in the cell viability at different concentrations of H2O2. The higher the concentration of H2O2, the lesser the viability of cardiomyocytes (Table 1).
| Concentration | ||
| Group (n = 8) | of H2O2 (mmol/L) | A570 nm (mean±SD) |
| Normal | 0 | 0.162±0.007 |
| 1 | 0.075 | 0.153±0.016 |
| 2 | 0.15 | 0.121±0.017a |
| 3 | 0.3 | 0.098±0.012b |
| 4 | 0.6 | 0.0798±0.006d |
Cardiomyocytes were exposed to a certain concentration of H2O2 and different concentrations of nm-haFGF. The total activity of SOD, MDA leakage, cellular viability and apoptosis were measured as the parameters for evaluating the protective effects of nm-haFGF. As shown in Table 2, the total activity of SOD dramatically increased with the concentration of nm-haFGF, the MDA leakages decreased with the concentration of nm-haFGF, and there was a significant difference between the control and experiment groups. As shown in Table 3, cellular viability increased with the increasing of concentration of nm-haFGF. All these showed that nm-haFGF had a significant cardiomyocytes protective effect on cardiomyocytes exposed to H2O2.
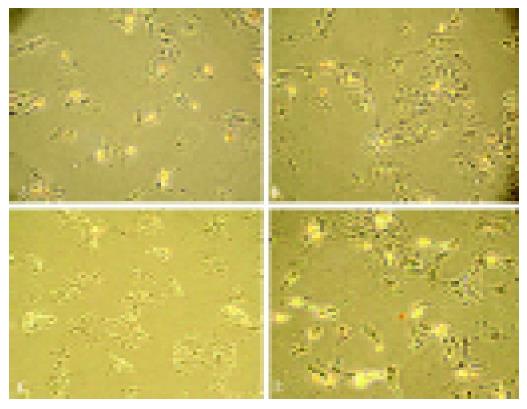
To examine the effect of H2O2 on apoptosis, myocytes were pretreated with different concentrations of H2O2. The initiating effect of H2O2 on cardiomyocytes apoptosis was confirmed by determining the change of DNA fragment (Figure 3). The cellular protective effect of nm-haFGF was confirmed by the same method (Figure 4). The rate of TUNEL-positive cells was 32.93.3% in the H2O2 group, and 1.10.26% in the normal group. But after being exposed to 0.15 mmol/L H2O2-DMEM, the rate of TUNEL-positive cells was 25.42.6% in 10 ng/mL nm-haFGF group, the cellular apoptosis rate in the 40 ng/mL nm-haFGF group was only 19.71.9%.
Studies indicate that intracoronary, intrapericardial or myocardial administration of FGFs, in chronically ischemic canine and porcine hearts, can stimulate angiogenesis, a process to minimize infarct size and improve cardiac function[11-13]. Although there are some promising results in the animal model trials, some questions, such as dose dependence[14], acute negative inotropic effects[10] still exist. Some investigators wondered if there would, be a potential danger of stimulating the growth of tumor for their strong power of mitogenesis[15]. In this study, we explored nm-haFGF responding to free radical-induced injury of primary cardiomyocytes when exposed to H2O2, which induces cardiac apoptosis[16,17]. As shown in Figure 2, when primary cultured neonatal SD mouse cardiac myocytes were exposed to different concentrations of hydrogen peroxide, a dramatic difference of morphological alteration was found, showing a concentration-dependent injury effect. The higher the concentration of H2O2, the more serious injury of the cardiomyocytes. As shown in Figure 3, at the lower concentration of H2O2, the cellular genomic DNA remained intact, when the concentration of H2O2 increased, cellular genomic DNA was divided into small fragments, suggesting that H2O2 has a strong cytotoxicity to primary cultured cardiac myocytes.
There is evidence that FGF family protein plays an important role in cardiac protection during hypoxia and reperfusion of ischemic myocardium[18-21]. It was reported that FGF should be considered as a protector rather than a promoter of myocardium regeneration in myocardium reperfusion injury[22-25].
In this study, we isolated and cultured the neonatal SD rat cardiomyocytes, built a cardiac myocyte injury model, and observed the cardio-protective effect of non-mitogenic human acidic FGF in vitro. We determined the cardiac myocytes injury by exposing it to different concentrations of H2O2, the cellular viability and the concentration of H2O2 showed a dose-dependent relationship. Cellular morphology and apoptosis examination showed that H2O2 had strong cytotoxicity to the cultured cardiomyocytes. nm-haFGF increased the total activity of SOD, protected the integrity of cellular membrane, and decreased the leakage of MDA as well as cell apoptosis, when cardiomyocytes were exposed to a certain concentration of H2O2. All these suggest that nm-haFGF can weaken the injury of cardiomyocytes in a dose-independent manner.
FGF has cardio-protective action on an isolated rat heart model[23,26,27]. Myocardial preconditioning can reduce infarct size, attenuate neutrophil infiltration, reduce calcium influx into cardiomyocytes, delay ultrastructural changes, and decrease programmed cell death (apoptosis)[23,28,29]. Therefore, one of the important mechanisms that can promote cardioprotection is by decreasing myocardial cell apoptosis identified in a wide variety of cardiovascular disorders, including myocardial infarction. As shown in Figure 3, when cardiomyocytes were exposed to a high concentration of H2O2, almost all the cells underwent apoptosis (Figure 2), and electrophoresis analysis showed the same results. When exposed to different concentrations of nm-haFGF, TUNEL assay showed that the rate of apoptosis dramatically decreased (Figure 4). All these indicate that nm-haFGF has the anti-apoptosis effect.
In conclusion, nm-haFGF has the anti-apoptosis effect at cell level.
The authors thank Dr. Tan Zhi (Physiological and Pathological Department, Sun Yat-Sen University) for his excellent technical support.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203-206. [PubMed] |
| 2. | Waltenberger J. Modulation of growth factor action: implications for the treatment of cardiovascular diseases. Circulation. 1997;96:4083-4094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Ishibashi Y, Urabe Y, Tsutsui H, Kinugawa S, Sugimachi M, Takahashi M, Yamamoto S, Tagawa H, Sunagawa K, Takeshita A. Negative inotropic effect of basic fibroblast growth factor on adult rat cardiac myocyte. Circulation. 1997;96:2501-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Forough R, Xi Z, MacPhee M, Friedman S, Engleka KA, Sayers T, Wiltrout RH, Maciag T. Differential transforming abilities of non-secreted and secreted forms of human fibroblast growth factor-1. J Biol Chem. 1993;268:2960-2968. [PubMed] |
| 5. | Jouanneau J, Plouet J, Moens G, Thiery JP. FGF-2 and FGF-1 expressed in rat bladder carcinoma cells have similar angiogenic potential but different tumorigenic properties in vivo. Oncogene. 1997;14:671-676. [PubMed] |
| 6. | Jouanneau J, Moens G, Thiery JP. The community effect in FGF-1 mediated tumor progression of a rat bladder carcinoma does not involve a direct paracrine signaling. Oncogene. 1999;18:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Cao DG, Li XK, Fu XB, Cheng B, Fang LJ. Effect of modified acidic fibroblast growth factor thymocyte apoptosis induced by dexamethasone in mouse. J China Biotechnol. 2003;23:80-84. |
| 8. | Goldspink PH, Thomason DB, Russell B. Beating affects the posttranscriptional regulation of alpha-myosin mRNA in cardiac cultures. Am J Physiol. 1996;271:H2584-H2590. [PubMed] |
| 9. | Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, O'Neill L, Cirielli C, Lakatta EG, Crow MT. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest. 1997;99:2635-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 227] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Nakamura TY, Goda K, Okamoto T, Kishi T, Nakamura T, Goshima K. Contractile and morphological impairment of cultured fetal mouse myocytes induced by oxygen radicals and oxidants. Correlation with intracellular Ca2+ concentration. Circ Res. 1993;73:758-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Wang GW, Schuschke DA, Kang YJ. Metallothionein-overexpressing neonatal mouse cardiomyocytes are resistant to H2O2 toxicity. Am J Physiol. 1999;276:H167-H175. [PubMed] |
| 12. | Waltenberger J. Modulation of growth factor action: implications for the treatment of cardiovascular diseases. Circulation. 1997;96:4083-4094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Sellke FW, Li J, Stamler A, Lopez JJ, Thomas KA, Simons M. Angiogenesis induced by acidic fibroblast growth factor as an alternative method of revascularization for chronic myocardial ischemia. Surgery. 1996;120:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Laham R, Simons M, Tofukuji M, Hung D, Sellke FW. Modulation of myocardial perfusion and vascular reactivity by pericardial basic fibroblast growth factor: insight into ischemia-induced reduction in endothelium-dependent vasodilation. J Theorac Cardiovasc Surg. 1998;116:1022-1028. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Baffour R, Berman J, Garb JL, Rhee SW, Kaufman J, Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemic: dose-response effect of basic fibroblast growth factor. J Vasc Surg. 1992;16:181-191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 206] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 535] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 17. | Forrest VJ, Kang YH, McClain DE, Robinson DH, Ramakrishnan N. Oxidant stress-induced apoptosis prevented by torolox. Free Radic Biol Med. 1994;16:675-684. [RCA] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038-5046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 286] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Jiang ZS, Srisakuldee W, Soulet F, Bouche G, Kardami E. Non-angiogenic FGF-2 protects the ischemic heart from injury, in the presence or absence of reperfusion. Cardiovasc Res. 2004;62:154-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Detillieux KA, Cattini PA, Kardami E. Beyond angiogenesis: the cardioprotective potential of fibroblast growth factor-2. Can J Physiol Pharmacol. 2004;82:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Safi J, DiPaula AF, Riccioni T, Kajstura J, Ambrosio G, Becker LC, Anversa P, Capogrossi MC. Adenovirus-mediated acidic fibroblast growth factor gene transfer induces angiogenesis in the nonischemic rabbit heart. Microvasc Res. 1999;58:238-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Horrigan MC, MacIsaac AI, Nicolini FA, Vince DG, Lee P, Ellis SG, Topol EJ. Reduction in myocardial infarct size by basic fibroblast growth factor after temporary coronary occlusion in a canine model. Circulation. 1996;94:1927-1933. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Horrigan MC, Malycky JL, Ellis SG, Topol EJ, Nicolini FA. Reduction in myocardial infarct size by basic fibroblast growth factor following coronary occlusion in a canine model. Int J Cardiol. 1999;68 Suppl 1:S85-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Hampton TG, Amende I, Fong J, Laubach VE, Li J, Metais C, Simons M. Basic FGF reduces stunning via a NOS2-dependent pathway in coronary-perfused mouse hearts. Am J Physiol Heart Circ Physiol. 2000;279:H260-H268. [PubMed] |
| 25. | Htun P, Ito WD, Hoefer IE, Schaper J, Schaper W. Intramyocardial infusion of FGF-1 mimics ischemic preconditioning in pig myocardium. J Mol Cell Cardiol. 1998;30:867-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Padua RR, Sethi R, Dhalla NS, Kardami E. Basic fibroblast growth factor is cardioprotective in ischemia-reperfusion injury. Mol Cell Biochem. 1995;143:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913-931. [RCA] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 512] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609-634. [PubMed] |
| 29. | Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 513] [Article Influence: 18.3] [Reference Citation Analysis (0)] |