Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5468
Revised: December 6, 2004
Accepted: December 9, 2004
Published online: September 21, 2005
AIM: To establish a highly reproducible animal model of acute liver failure (ALF), for assessing the effect of bioartificial liver support system (BALSS).
METHODS: A two-phase complete liver devascularization procedure was performed in eight loco-hybrid pigs. Blood biochemical index and liver biopsy were studied every 2 h after surgery, and survival time was recorded. The BALSS constructed with high volume recirculating technique was a hollow fiber circulating system consisting of a hepatocyte reactor-hollow fiber module inoculated with microcarrier-adhering hepatocytes, and a double pump, heparinized, thermostabilized, micro-capsulized activated carbon-adsorbing plasmapheresis system. Twelve pigs undergoing two-phase surgery were randomized into: control group (perfused without hepatocytes, n = 6) and treatment group (perfused with hepatocytes, n = 6). Intergroup liver biochemical indexes, survival time, and liver pathological changes were analyzed at regular intervals.
RESULTS: Two-phase surgery was performed in all the experimental pigs, and there was no obvious difference between their biochemical indexes. After 3 h of phase II surgery, ammonia (Amm) increased to (269±37) μmol/L. After 5 h of the surgery, fibrinogen (Fib) decreased to (1.5±0.2) g/L. After 7 h of the surgery, ALT, AST, Tbil and PT were (7.6±1.8) nka/L, (40±5) nka/L, (55±8) μmol/L and (17.5±1.7) nka/L respectively. After 9 h of surgery, ALB and Cr were (27±4) g/L and (87±9) μmol/L. After 13 h of surgery, BUN was (3.5±0.9) μmol/L. All the above values were different from those determined before surgery. Survival time of pigs averaged 13.5±1.4 h. ALF pigs in the other group were treated with BALSS. The comparison analysis between the treated and control animals showed the changes of Tbil, PT, Alb, BUN, Cr, Fib, and Amm (P<0.01), but there was no change of ALT and AST. The survival time was statistically different (P<0.01), and there was no significant difference in histological changes.
CONCLUSION: The porcine ALF model established by two-phase devascularized surgery is valid and reproducible. The hollow fiber BALSS can meet the needs of life support and is effective in treating ALF.
- Citation: Gao Y, Mu N, Xu XP, Wang Y. Porcine acute liver failure model established by two-phase surgery and treated with hollow fiber bioartificial liver support system. World J Gastroenterol 2005; 11(35): 5468-5474
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5468.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5468
A highly reproducible animal model of ALF is crucial for assessing the extracorporeal BAL support efficiency. In our investigation, when a two-phase complete liver devascularization surgery was performed, acute loss of compensation for liver functions occurred, and pigs died of acute liver failure (ALF) eventually. Blood samples were collected to analyze biochemical indexes and liver biopsy was obtained for pathologic examinations under light and electron microscope. The survival time of all experimental animals was recorded. Pigs with ALF were treated with the bioartificial liver support system (BALSS) consisting of a hepatocyte reactor-hollow fiber module inoculated with microcarrier-adhering hepatocytes, and a double pump, heparinized, thermostabilized, micro-capsulized activated carbon-adsorbing plasmapheresis system. Pigs with ALF in the other group were treated with BALSS. Compared to the control animals, Tbil, PT, Alb, BUN, Cr, Fib, and Amm in the treated animals changed (P<0.01), but ALT and AST did not change. Survival time was statistically different (P<0.01), but there was no difference in histological changes.
Twenty 2-3 mo old healthy native hybrid pigs (15-20 kg) purchased from the Animal Center of Sun Yat-Sen University of Medical Sciences were used in the experiment. All the animals received humane care and the study protocols were in compliance with the animal care guidelines established by the First Military Medical University. Twelve grams per liter alginic acid sodium reservoir, 13 g/L CaCl2 solution, poly-lysine solution, and 0.6 g/L alginic acid sodium solution were kindly provided by the Institute of Molecular Immunology, The First Military Medical University. All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Machines used included Fresenius hemodialysis machine (A2008C, Germany), UF5.5F6 dialyzer (Fresenius Polysulfone, Germany), YT-HP-160 hemoperfusion machine (Yatai Medical Instrumentation Co., Ltd, Ningbo, Zhejiang Province, China).
Prior to surgery, animals were kept fasting for 12 h, with water available ad libitum. Anesthesia was induced with an intra-abdominal bolus of 30 mg/kg pentobarbital (30 g/L). A double lumen catheter was inserted into the right upper limb vein for drug and infusion delivery. Animals received oxygen through nasal catheter intubation. Laparotomy was performed in midline from xiphoideus down to the lower abdomen. After mobilization of the portal vein and right renal vein, 0.25 mg heparin was injected into the portal vein, which was clamped in the middle part with a hemostat, a 15 cm×0.8 cm silica-gel catheter containing 20 g/L heparin saline was introduced into the inferior caval vein with a depth of 2-3 cm from the far-forth end of the right renal vein, and secured. Another end of this catheter was placed in the portal vein toward the far-forth end with a depth of 1.5 cm, then the catheter was secured and opened. When there was no distinct bleeding, the abdominal wound was closed. Thus a portacaval shunt was established end to side. Phase II surgery was performed after 2 d. All the peri-hepatic ligaments were cut. The branches of hepatic artery were transfixed; hepatic artery and gastroduodenal artery were ligated completely. An 18-G catheter was inserted into the left femoral artery for direct blood pressure measurement. After 1, 4, and 7 h of complete liver devascularization, liver biopsies were taken for postoperative histology examination. The operative wound was closed in a single layer. During the surgery, 500 mL 0.9% NaCl and 800 000 U benzylpenicillin were infused. When the animal died, autopsy was practiced, and specimens of liver were taken for histology examination.
Biochemical index assessment During phase II surgery, blood sample was collected before the obstruction of blood liver (0 h) and at regular intervals (every 2 h after phase II surgery) to monitor AST, ALT, Alb, Tbil, Amm, BUN, Cr, PT, and Fib until the pigs died.
Histology examination Under light microscope, liver specimens were trimmed into 5-mm sections, fixed into 100 mL/L formalin, dehydrated with alcohol, embedded with paraffin and sliced up, then stained with HE for observation under light microscope. Under electronic microscope, liver specimens were doubly-fixed with 25 g/L glutaral and 10 g/L osmium acid, dehydrated with acetone, embedded with Epon-812 resin and sliced up by LKB-2088 ultramicrotome, then stained with acetic acid and citromalic acid lead for electronic microscopy with Hitachi-600 transmission electron microscope (Japan).
Survival time record Survival time (from phase II surgery to the death of animal) of all the animals was recorded.
Porcine hepatocyte preparation Hepatocytes of native hybrid pigs were isolated by the two-step collagenase digestion method[1]. The digested liver parenchyma was suspended in ice cold Hanks-Hepes medium and filtered with gauze and 500 μm of stainless steel mesh. Then, the cell pellet was collected by centrifugation at 50 r/min and washed several times with Hanks-Hepes balanced solution. Viability of the cells was always greater than 90%, as judged by trypan blue exclusion. Isolated liver cells were attached to hydrated microcarriers according to the methods of Demetriou et al[2].
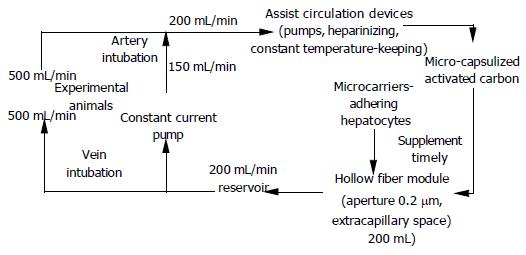
Construction of BAL circulatory system Micro-capsulization of activated carbon was conducted according to the method of Rozga et al[3]. (Figure 1).
System parameters Blood was pumped out from the femoral catheter at a flow rate of 50 mL/min and into a high flux recirculation circuit, and then converged with the recircling blood at a flow rate of 150 mL/min, which was pumped out of the reservoir by constant current pump. The confluent blood was filtrated through the double pumps, heparinizing, thermostabilized, oxygenating, pressure monitoring and micro-capsulized charcoal adsorption devices in order, and then passed through the intracapillary space of the hollow fiber module at 200 mL/min. The extracapillary space of the device was filled with 200 mL of microcarrier-adhering hepatocytes and culture media system (containing 5×109 hepatocytes). Solute exchange occurred through the side pore of the fiber. Subsequently, 500 mL of the circling blood flowed into the reservoir and a majority of it (150 mL/min) turned into recirculation through the pump. The rest (50 mL/min of blood) was infused back into the pigs through the femoral vein catheter.
Treatment of ALF with BALSS Twelve experimental pigs were randomized into control group and treatment group (n = 6 in each group). When construction of BALSS was completed, the groups with ALF were treated with BALSS. The control animals were treated with BALSS containing no hepatocytes, but only 200 mL of culture medium, and the treatment group was treated with BALSS containing 5×109 hepatocytes in the same volume of culture medium. Analysis of liver biochemical indexes, survival time and morphological changes was carried out to judge the extracorporeal liver support efficiency of BALSS.
Results were expressed by mean±SD. Data were analyzed by the t test. P<0.05 was considered statistically significant.
Experimental ischemic ALF model was established successfully in eight pigs by two-phase complete liver devascularization surgery. After the obstruction, the average survival time was (13.5±1.4) h.
Biochemical assays After devascularization, Amm increased immediately, followed by a decrease after 9-11 h, and then increased progressively again. Levels of AST, ALT, Tbil, BUN, Cr, and PT increased. Alb and Fib decreased (Table 1).
| 0 h | 1 h | 3 h | 5 h | 7 h | 9 h | 11 h | 13 h | |
| ALT (nkat/L) | 4.7±0.7 | 5.1±1.3 | 4.9±1.2 | 5.2±1.5 | 7.6±1.8a | 8.2±2.1a | 10.9±3.4a | 16.2±1.2a |
| AST (nkat/L) | 18±1 | 19±1 | 20±1 | 25±2 | 40±5a | 113±22a | 30±8a | 10±4a |
| Tbil (μmol/L) | 40±6 | 44±9 | 41±7 | 47±10 | 55±8a | 49±8a | 52±5a | 59±10a |
| Alb (g/L) | 36±5 | 33±4 | 31±4 | 30±4 | 28±3 | 27±4a | 27±3a | 25±4a |
| Amm (μmol/L) | 117±18 | 215±29 | 269±37a | 285±34a | 275±30a | 239±28a | 216±19a | 280±35a |
| BUN (mmol/L) | 2.7±0.6 | 2.8±0.4 | 2.5±0.4 | 2.5±0.6 | 2.8±0.5 | 2.8±0.4 | 2.9±0.5 | 3.5±0.9a |
| Cr (μmol/L) | 74±8 | 77±7 | 77±9 | 64±5 | 63±7 | 87±9a | 102±17a | 98±10a |
| PT (s) | 12.8±1.0 | 13±1.1 | 14.4±1.4 | 15.4±1.8 | 17.5±1.7a | 19.2±1.9a | 21.3±1.9a | 21.9±2a |
| Fib (g/L) | 2.2±0.2 | 2.1±0.2 | 1.9±0.2 | 1.5±0.2a | 1.2±0.2a | 0.8±0.1a | 0.8±0.1a | 0.7±0.1a |
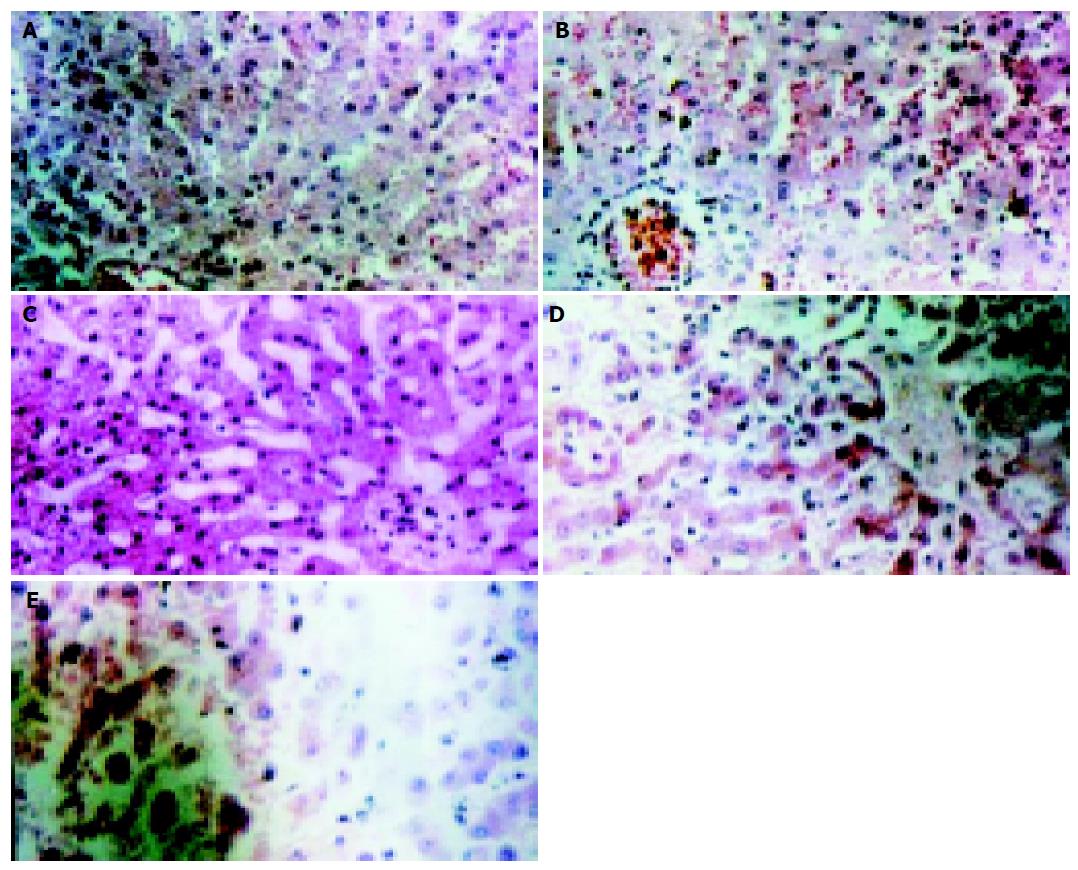
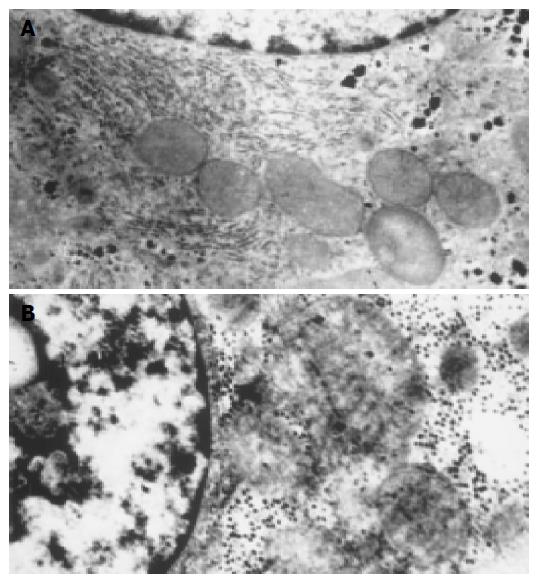
Histology observation Light microscopy observation: Before the complete liver devascularization (0 h), structure of hepatic lobules was normal, Disse cavity did not dilate, hepatocytes did not swell, and arrayed radiatively around the central vein (Figure 2A). One hour later, structure of hepatic lobules was still normal, so was the linage of hepatocytes. Disse cavity dilated slightly, and hepatocytes were swollen and turbid, while nuclear membrane and nucleoli were still clear (Figure 2B). Four hours later, structure of hepatic lobules was still in existence, so was the frame of hepatic plates. While Disse cavity dilated obviously, central veins caved in and ballooning degeneration occurred in cytolymph. Pyknosis and vanishment of nucleoli occurred as well (Figure 2C). Seven hours after the obstruction, structure of hepatic lobules was in disorder, hepatic plate was dissociated and the arrangement of hepatocytes was disordered, Disse cavity dilated more, ballooning degeneration in cytolymph was more significant, so was the swelling of hepatocytes, chromatin gathered borderly, and there was evidence of lytic necrosis of hepatocytes (Figure 2D). When the experimental animals died of ALF, pathological examinations revealed that hepatic lobules were unrecognized, hepatocytes diminished, hepatic plate dissociated, and obvious extensive piecemeal necrosis of hepatocytes emerged (Figure 2E). Electronic microscopy observation: Before devascularization, the examination of hepatocyte ultrastructures showed that there was abundant glycogen granules in cytolymph, also plenty of endoplasmic reticulum with no dilation and no swelling mitochondria, and mitochondrial cristae were clear, so was mitochondrial membrane, chromatin was symmetrical, nucleoli and nuclear membrane were also legible (Figure 3A). Four hours later, it was obvious that glycogen granules in cytolymph were homogenized, rough endoplasmic reticulum de-grained, mitochondrion was swollen, and part of it was lysed, mitochondrial cristae blurred, cholangiole microvilli between the hepatocytes significantly diminished, chromatin centralized, nuclei deformed, and pseudo-inclusion bodies in nuclei appeared (Figure 3B).
Experimental ischemic ALF model was established successfully in 12 pigs by the two-phase complete liver devascularization surgery, and treatment of all the pigs with BALSS was accomplished successfully.
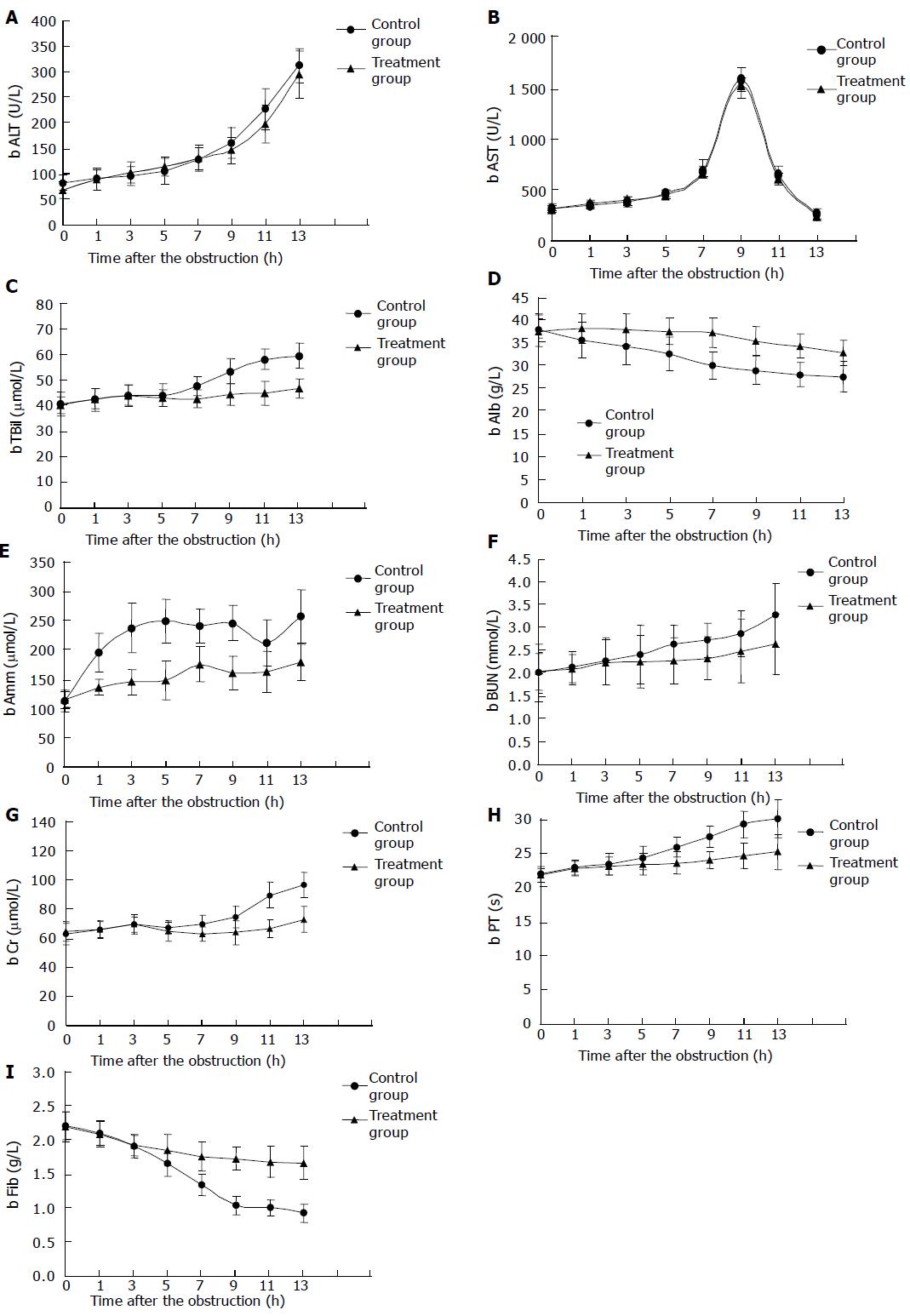
Determination of biochemical indexes Before the complete liver devascularization, the difference in ALT and AST had no statistical significance between the two groups; after the surgery, ALT and AST increased progressively and swiftly in both groups after the surgery, but there was no statistical significance between the two groups (Figures 4A and B). A significant difference in Tbil level emerged between the two groups after 7 h (P<0.05), this difference became more significant after 9 h (P<0.01, Figure 4C). The significant difference in Alb between the two groups emerged after 3 h (P<0.05) and became more significant 2 h later (P<0.01, Figure 4D). Amm level increased rapidly in the control animals, but slowly in the treated animals, the difference was significant (P<0.01, Figure 4E). The levels of BUN and Cr increased more slowly in both groups as compared to Amm; the difference between the two groups was observed after 5 h (P<0.05), and became more significant 7 h later (P<0.01, Figures 4F and G). There was no statistical significant difference in PT and Fib between the two groups after 3 h, but the difference in PT tended to be significant 5 h later (P<0.05), and difference in Fib was more significant (P<0.01, Figures 4H and I).
Morphology observation Liver pathological impairment in the two groups was analogous to the pathological images of ALF model.
Survival time Survival time of the control animals was 11.6-15.1 (averaged 13.17±1.47) h, and that of the treated animals was 18.1-24.4 (averaged 21.33±2.16) h. The difference was significant between the two groups (P<0.01).
Hepatotoxins, such as alcohol, anaflon, dimethyl-nitramine and D-galactosamine, are used as ALF chemical inducers. But the chemical ALF models induced by hepatotoxin can have much diversity in the changes of biochemical indexes, pathological examinations, mortality rate, and survival time. Thus, it is hard to evaluate the effects of treatments in those models. Also hepatotoxins often impair the internal organs such as kidneys and lungs, thus complicating the causes of death. Surgical ALF models are preferred, especially when a large animal model is established. Since devascularization method to induce canine ALF with two-phase surgery was reported by Rapaport and Gigesin, 1953, the procedure has been modified by the technique of hepatic artery ligation[4-6].
In our experiment, two-phase surgery was performed for liver devascularization. The pigs underwent portal-right renal venous shunt, and 2 d later, hepatic artery, gastroduodenal artery and peri-hepatic ligaments were cut to obstruct blood flowing into the liver. Acute loss of compensation for liver functions was then produced and the pigs died of ALF. We found that this model was simple and easy to operate. The choice of portal-right renal venous intubation shunt instead of portal-cava anastomosis reduced the difficulty of the surgery greatly. The blood flowing into liver was obstructed in phase I surgery, which induced ischemia tolerance of the pig. Thus, all the experimental pigs could accept phase II surgery. The model was reproducible. Stable parameters like biochemical indexes and survival time could be obtained, and the success rate of surgery was high. The two-phase surgery could achieve devascularization of liver, while not abruptly diminishing the blood volume in heart, the function of other internal organ was not influenced greatly. The pathologic changes in this model were irreversible, just like the model of total hepatectomy. The key technical point of this ALF model is as follows: The liver rami of left gastric artery and diaphragm artery must be ligated thoroughly during phase II surgery. Because the anatomic structure of porcine liver is similar to that of human beings, the same procedures to obstruct liver rami of human peri-hepatic ligaments were adopted in this study. Portocaval anastomosis is advantageous over portocaval intubation shunt theoretically, but in practice, portocaval intubation shunt is simple and reliable. In the prophase experiments of our study, the metabolism indexes of normal pigs were compared to those of the pigs with their right renal vein ligated. The results indicated that there was no significant difference in Amm, BUN, Cr, and blood sugar fluctuation between the two groups. The time of portocaval intubation shunt was much shorter than that of portocaval anastomosis (just about 5 min). No interference with hemodynamics of portal vein was found in the cases of right renal venous cannulation. The color and luster of bowel were ruddy during phase II surgery, indicating that 0.8-cm caliber of the shunt catheter is advisable.
ALF is a clinical syndrome involving massive hepatocyte necrosis, and resulting in liver function failure and encep-halopathy. The death rate of ALF can reach 80%, and there is no specific therapy for it. The introduction of liver transplantation into clinical practice has improved the survival time of patients. However, because of the deficiency in donor liver, about 50% of the ALF patients would die waiting for donors. Since liver has a powerful ability to regenerate, an effective artificial liver assembly has been established to replace the liver functions of ALF patients for a temporary period, till the liver itself regenerates and recovers from acute decompensation. Recently, with advances in cell engineering and material technology, various kinds of large-scale cell culture techniques are used for the study of bioartificial liver, such as microcarriers[7,8], microcapsule coating[9], gelatin embedding[10-12], hollow fiber module[13-15] and spheroid culture[16], etc. The BALSS of porcine hepatocyte reactor-hollow fiber module was established and has entered into phase I clinical trials. It has been proven that such a system can yield multifunctions of hepatocytes to support the patients with ALF passing over the crisis. The BALSS can serve as a bridge to liver transplantation[14-18].
The technical characteristics of BALSS are as follows: (1) The microcarrier-adhering hepatocytes and culture media system increase the adsorbing area of hepatocytes greatly. Sufficient contact of cell-matrix or cell-cell is ensured. It is quite helpful to maintain hepatocytes polarity, thus prolonging the stability of hepatocytes. (2) In the circulation loop, high volume recirculating technique is adopted, which facilitates liquor convection greatly, and improves the substrate-product exchange of hepatocytes and the efficiency of BAL circulation. (3) Before the blood is pumped into the hepatocyte reactor, hemoperfusion over charcoal is used to relieve the hepatocyte impairment induced by the toxic substances in blood, thus hepatocyte viability and function can be maintained longer[19,20]. In this study, after devascul-arization of the liver, ALT increased progressively and swiftly in both groups, but there was no statistical significance between the two groups, suggesting that the degree of hepatocyte impairment is similar in both groups. Since devascularization of the liver in this study was permanent, the impairment of liver function was irreversible. AST could not be determined in treated animals 15 h later. The reason is likely that hepatocyte mitochondria which could not endure hypoxia for a long time reach the peak of disruption about 9 h later, and AST is exhausted gradually. After the experimental pigs died, liver pathological impairment of the two groups was analogous to the pathological image of ALF models, suggesting that the model of ALF established in this study is stable.
During the treatment of BAL, the most impressive result was the fluctuation of Amm. Amm is mainly produced by intestinal bacteria and detoxificated through being synthesized into urea in liver. After the obstruction, Amm level in control animals increased rapidly from (110±17) μmol/L to (240±35) μmol/L 5 h later, while increased slowly from (112±14) μmol/L to (144±34) μmol/L in the treated animals (P<0.01), suggesting that the BALSS can detoxify and depress Amm. Similar results have been reported by Chen et al. The levels of Tbil, Alb, BUN, Cr, PT, and Fib were significantly different between the two groups 3-7 h later, suggesting that BALSS has some action on bilirubin metabolism.
In conclusion, the BALSS can be used in the treatment of ALF. The results achieved in this study of large animals may contribute to the clinical therapy of ALF with BALSS.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Seglen PO. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973;82:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 946] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, Kram M, Chowdhury JR. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233:1190-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 203] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Rozga J, Holzman MD, Ro MS, Griffin DW, Neuzil DF, Giorgio T, Moscioni AD, Demetriou AA. Development of a hybrid bioartificial liver. Ann Surg. 1993;217:502-509; discussion 502-509;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Rivas-Vetencourt PA, Aranda ED, Sorio L, Quero Z, Martinez A, Vegas AM, Zerpa MJ. Xenotra-nsplantation of isolated encapsulated porcine hepatocytes in the treatment of a highly fulminant hepatic failure model. Transplant Proc. 1997;29:920-922. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Dixit V, Gitnick G. The bioartificial liver: state-of-the-art. Eur J Surg Suppl. 1998;582:71-76. [PubMed] |
| 6. | Ryska M, Kieslichová E, Pantoflícek T, Ryska O, Zazula R, Skibová J, Hájek M. Devascularization surgical model of acute liver failure in minipigs. Eur Surg Res. 2004;36:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Werner A, Duvar S, Müthing J, Büntemeyer H, Lünsdorf H, Strauss M, Lehmann J. Cultivation of immortalized human hepatocytes HepZ on macroporous CultiSpher G microcarriers. Biotechnol Bioeng. 2000;68:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Morsiani E, Pazzi P, Moscioni AD, Rozga J, Azzena G, Demetriou AA. In vitro morphological and functional characterization of isolated porcine hepatocytes for extracorporeal liver support: bile acid uptake and conjugation. J Surg Res. 1998;79:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Yin C, Mien Chia S, Hoon Quek C, Yu H, Zhuo RX, Leong KW, Mao HQ. Microcapsules with improved mechanical stability for hepatocyte culture. Biomaterials. 2003;24:1771-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Naka S, Takeshita K, Yamamoto T, Tani T, Kodama M. Bioartificial liver support system using porcine hepatocytes entrapped in a three-dimensional hollow fiber module with collagen gel: An evalution in the swine acute liver failure model. Artif Organs. 1999;23:822-828. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Suh KS, Lilja H, Kamohara Y, Eguchi S, Arkadopoulos N, Neuman T, Demetriou AA, Rozga J. Bioartificial liver treatment in rats with fulminant hepatic failure: effect on DNA-binding activity of liver-enriched and growth-associated transcription factors. J Surg Res. 1999;85:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Rivera DJ, Gores GJ, Misra SP, Hardin JA, Nyberg SL. Apoptosis by gel-entrapped hepatocytes in a bioartificial liver. Transplant Proc. 1999;31:671-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Hay PD, Veitch AR, Smith MD, Cousins RB, Gaylor JD. Oxygen transfer in a diffusion-limited hollow fiber bioartificial liver. Artif Organs. 2000;24:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Patzer JF, Mazariegos GV, Lopez R, Molmenti E, Gerber D, Riddervold F, Khanna A, Yin WY, Chen Y, Scott VL. Novel bioartificial liver support system: preclinical evaluation. Ann N Y Acad Sci. 1999;875:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Chung YJ, Lee HJ, Koh YT, Kim SB, Kim SH, Choi SH, Yi NJ, Chang SH, Yang EL, Suh KS. [Isolation and culture of pig hepatocyte in large scale for the application of bioartificial liver system]. Taehan Kan Hakhoe Chi. 2002;8:249-255. [PubMed] |
| 16. | Maddrey WC. Bioartificial liver in the treatment of hepatic failure. Liver Transpl. 2000;6:S27-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Nyberg SL, Hay EJ, Ramin KD, Rosen CB. Successful pregnancy after porcine bioartificial liver treatment and liver transplantation for fulminant hepatic failure. Liver Transpl. 2002;8:169-170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Tsiaoussis J, Newsome PN, Nelson LJ, Hayes PC, Plevris JN. Which hepatocyte will it be? Hepatocyte choice for bioartificial liver support systems. Liver Transpl. 2001;7:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Flendrig LM, Chamuleau RA, Maas MA, Daalhuisen J, Hasset B, Kilty CG, Doyle S, Ladiges NC, Jorning GG, la Soe JW. Evaluation of a novel bioartificial liver in rats with complete liver ischemia: treatment efficacy and species-specific alpha-GST detection to monitor hepatocyte viability. J Hepatol. 1999;30:311-320. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Baquerizo A, Mhoyan A, Kearns-Jonker M, Arnaout WS, Shackleton C, Busuttil RW, Demetriou AA, Cramer DV. Characterization of human xenoreactive antibodies in liver failure patients exposed to pig hepatocytes after bioartificial liver treatment: an ex vivo model of pig to human xenotransplantation. Transplantation. 1999;67:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |