Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5412
Revised: April 13, 2005
Accepted: April 18, 2005
Published online: September 14, 2005
We report a patient with HBV-related hepatocellular carcinoma (HCC) and refractory ascites who had received a peritoneal-venous shunt (PVS) 1 year before liver transplantation. Urgent surgical intervention following bowel obstruction and failure of immunosuppression therapy. No intestinal obstruction was found during an initial PVS. However, intestinal obstruction developed 2 wk after liver transplantation; and a cocoon abdomen was found upon exploration. This is the first reported case of cocoon abdomen caused by PVS and exacerbated by liver transplantation.
- Citation: Lin CH, Yu JC, Chen TW, Chan DC, Chen CJ, Hsieh CB. Sclerosing encapsulating peritonitis in a liver transplant patient: A case report. World J Gastroenterol 2005; 11(34): 5412-5413
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5412.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5412
Cocoon abdomen or sclerosing encapsulating peritonitis (SEP) is a rare cause of small bowel obstruction. In this condition, the bowel is partially or totally encased or wrapped by a thick fibrous membrane forming several compartments (cocoons) containing loops of small bowel. SEP usually presents with symptoms of intestinal obstruction, and pre-operative diagnosis is difficult. The pathogenesis of this condition remains unclear; however, it is a form of chronic irritation and inflammation, and may be summarized as primary (idiopathic) or secondarily induced[1]. Here we report a patient with liver cirrhosis and massive ascites who had received a peritoneal-venous shunt (PVS) 1 year before liver transplantation. Thickened parietal peritoneum and fibrotic changes were found during the liver transplantation. After surgery, the patient received treatment with high-dose immunosuppressive agents. Intestinal obstruction developed 2 wk later and this was resolved by surgery.
A 64-year-old man received a liver transplantation on August 10, 2003, for decompensated end-stage liver disease caused by hepatitis B, with liver cirrhosis and hepatoma. His pre-transplant history included receiving transarterial embolization of the hepatocellular carcinoma, (HCC) 2 wk before transplantation and a PVS drainage, 1 year before liver transplantation for treating refractory ascites. After the PVS had been performed, the ascites was controlled and the patient was feeding well.
Before liver transplantation, the patient’s laboratory data were albumin 3.4 g/dL, total bilirubin 1.9 mg/dL, and prothrombin time 12.3/11.2 s. His Child-Pugh classification was B (score, 8), and he had no history of hepatic encep-halopathy. During the liver transplantation, the parietal peritoneum and most of the colonic wall and retroperitoneal liver were observed to be thickened and fibrotic. Hydrocorti-sone Sod. Succinate (Solu-cortef) (1 000 mg) was administered. After liver transplantation, the patient started to receive immunosuppressive treatment, including Prograf (1 mg twice daily), Solumedrol (40 mg four times daily), CellCept (500 mg twice daily), and we maintained the therapeutic level of Prograf at 10-12 ng/mL. The patient’s oral feeding condition was poor; he could not even tolerate semi-liquid diet.
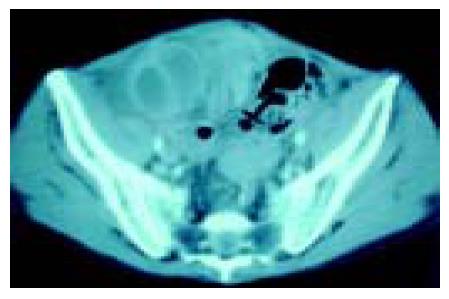
Two weeks after liver transplantation, colicky abdominal pain, vomiting and constipation developed, but no rebounding pain was found by physical examination. Contrasted abdominal computer tomography (CT) revealed a dilated C-shaped region of small intestine over the right lower pelvis, and an adhesion band was suspected to have caused a closed-loop obstruction (Figure 1). Conservative treatment was started, but little improvement occurred and the patient still could not tolerate oral feeding. The patient’s abdominal distension increased; repeated plain abdominal radiography showed that the obstructed small bowel loops were enlarged, so we decided on emergency surgery.
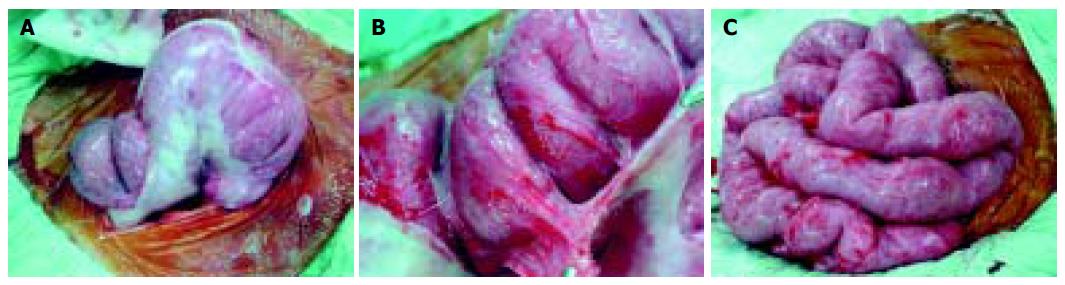
At laparotomy, we found dense, thick, adhesive sheaths circumferentially wrapping loops of small bowel giving the shape of cocoons in the abdomen (Figure 2). The loops within each cocoon were normal and adherent to each other with avascular fine fibrous tissue; this was easily broken down with finger dissection. The serosa of the bowel was intact. The patient’s post-operative recovery was uneventful, and he was discharged 2 wk later.
SEP involves entrapment of the intestine in a fibrous sac, resulting in complete intestinal obstruction. It was first described in 1907 by Owtschinnikow[2]. The real causes of SEP are unclear, but it can be classified as primary and secondary forms[1]. Primary (idiopathic) SEP occurs in young women living in warm climates, and is thought to be caused by retrograde menstruation or infection via the Fallopian tubes[3]. Secondary SEP can be classified as systemic caused by local irritants, or as local combined with systemic irritants. The major possible causes include long-term use of β-adrenergic blocking agents, chronic ambulatory peritoneal dialysis, and liver cirrhotic patients after PVS[1,4-6].
If diagnosed before obstruction occurs, the sclerotic membrane can frequently be separated from the small intestine. Successful separation is almost impossible when associated with complete obstruction. Thus, early diagnosis is important.
The clinical picture of cocoon abdomen usually includes recurrent episodes of intestinal obstruction caused by kinking and compression of the intestines within the constricting cocoon. The condition should be suspected in patients with bowel obstructive signs or soft, non-tender abdominal masses. Pre-operative diagnosis of abdominal cocoon is difficult. Plain radiographs and oral contrast studies may show a circumscribed mass of bowel loops with absent or delayed passage of contrast. Ultrasound examination may show a mass of tightly bound small bowel loops surrounded by a thick rim of echo-poor tissue. CT scans might also reveal a mass comprising an encapsulating cluster of dilated small bowel loops with failure of orally taken contrast medium to pass distally.
Beta-blockers, particularly propranolol, have been implicated in the etiology of this condition because they may lead to enhanced collagen production and subsequent fibrosis. Yamamoto et al[5] reported SEP in two patients with liver cirrhosis. Cambria et al and Greenlee et al[2] reported possible associations with the Le Veen shunt. Abul reported the first case of cocoon abdomen in a liver transplant patient, but that patient did not receive the Le Veen shunt.
Our patient suffered from liver cirrhosis with massive ascites and received PVS, but he presented with SEP following liver transplantation and developed intestinal obstruction 2 wk later. Although the actual pathogenesis of SEP is unclear, we believe that PVS may be one of the causes. Thus, ascites flow through the PVS and fibrin deposition in the peritoneum may lead to chronic inflammation. Hence, the cause of SEP in this patient may have been a combination of PVS with recurrent spontaneous bacterial peritonitis. Intraperitoneal bleeding may have helped induce this condition. Following liver transplantation, bleeding and manipulation irritation would have exacerbated it[5].
Definitive diagnosis of SEP can only be made at laparotomy. Yamamoto reported successful treatment with total enterolysis and the oral administration of prednisolone[5], and Junor and McMillan reported that immunosuppression has a beneficial effect on SEP in patients with or without a successful transplantation. However, in this patient, even large doses of immunosuppressants were ineffective. Hence, surgery was essential with lysis of the membranes and adhesions. Bowel resection was not necessary. We recommend that SEP should be cleared away during liver transplantation to prevent any following possible intestinal obstruction.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Cohen O, Abrahamson J, Ben-Ari J, Frajewicky V, Eldar S. Sclerosing encapsulating peritonitis. J Clin Gastroenterol. 1996;22:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Abul S, Al-Oazweni H, Zalat S, Al-Sumait B, Asfar S. Cocoon abdomen in a liver transplant patient. J R Coll Surg Edinb. 2002;47:579-581. [PubMed] |
| 3. | Narayanan R, Bhargava BN, Kabra SG, Sangal BC. Idiopathic sclerosing encapsulating peritonitis. Lancet. 1989;2:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Brown P, Baddeley H, Read AE, Davies JD, McGarry J. Sclerosing peritonitis, an unusual reaction to a beta-adrenergic-blocking drug (practolol). Lancet. 1974;2:1477-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 165] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Yamamoto S, Sato Y, Takeishi T, Kobayashi T, Hatakeyama K. Sclerosing encapsulating peritonitis in two patients with liver cirrhosis. J Gastroenterol. 2004;39:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Klimopoulos S, Katsoulis IE, Margellos V, Nikolopoulou N. Sclerosing encapsulating peritonitis secondary to CAPD: the effect of fibrotic debridement on further dialysis. J R Coll Surg Edinb. 2002;47:485-490. [PubMed] |