Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5381
Revised: March 20, 2005
Accepted: March 24, 2005
Published online: September 14, 2005
AIM: To develop a Brown Norway (BN) rat model to determine the potential allergenicity of novel proteins in genetically modified food.
METHODS: The allergenicity of different proteins were compared, including ovalbumin (OVA), a potent respiratory and food allergen, bovine serum albumin (BSA), a protein that is considered to have a lesser allergenic potential, and potato acid phosphatase (PAP), a non-allergenic protein when administered to BN rats via different routes of exposure (intraperitoneally or by gavage). IgG and IgE antibody responses were determined by ELISA and PCA, respectively. An immunoassay kit was used to determine the plasma histamine level. In addition, possible systemic effect of allergens was investigated by monitoring blood pressure.
RESULTS: OVA provoked very vigorous protein-specific IgG and IgE responses, low grade protein-specific IgG and IgE responses were elicited by BSA, while by neither route did PAP elicit anything. In either routes of exposure, plasma histamine level in BN rats sensitized with OVA was higher than that of BSA or PAP. In addition, an oral challenge with BSA and PAP did not induce any effect on blood pressure, while a temporary drop in systolic blood pressure in few animals of each routes of exposure was found by an oral challenge with OVA.
CONCLUSION: BN rat model might be a useful and predictive animal model to study the potential allergenicity of novel food proteins.
- Citation: Jia XD, Li N, Wu YN, Yang XG. Studies on BN rats model to determine the potential allergenicity of proteins from genetically modified foods. World J Gastroenterol 2005; 11(34): 5381-5384
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5381
Modern biotechnology has resulted in the introduction of a number of new proteins to foods, the potential allergenicity of these new proteins is a major concern in the safety assessment on genetically modified foods. The best known systematic approach for the assessment of the allergenic properties of novel proteins was called IFBC/ILSI decision tree, which was jointly developed by the International Food Biotechnology Council (IFBC) and the International Life Sciences Institute (ILSI)[1]. A universal, reliable and relevant in vitro or in vivo test to study the sensitizing potential of a new protein is, however, not available. Therefore, it is recommended that the development and validation of a widely accepted animal model should be the most direct approach to determine the sensitizing potential of a new protein. In 2001, FAO/WHO revised this decision tree and developed another one called FAO/WHO 2001 decision tree, in which animal models were included[2].
An ideal animal model should satisfy the following important criteria: sensitization and challenge should preferably be oral; preferably no use of adjuvants; the test animal should produce a significant amount of IgE antibody; the test animal should tolerate most food proteins; clinical reactions should be similar to those seen in humans; antibody responses should be directed to similar proteins as found in patient sera; and the model should be relatively easy to conduct and reproducible both in time and in different laboratories[3]. Several attempts have been made to develop animal models for food allergy research, mainly in mouse, guinea pig, and rats[4-6]. Some drawbacks limited the further use of the guinea pig in food allergy research, including significant differences in immunophysiology, limited knowledge of its immune system, and lack of tools to study its immune system. Studies in mice on oral protein administration without adjuvants readily resulted in tolerance induction[7]. For the rat, there are some advantages including the most commonly used species in toxicity testing, a reasonable amount of knowledge on its immune system, and many tools for immune-related studies. Studies have showed that the Brown Norway (BN) rat is a high-immunoglobulin (particularly IgE) responder strain[8,9].
There is some debate regarding the most appropriate route of administration. In theory, oral administration appears to be the most attractive and relevant; however, it is well known that exposure of rats to proteins in this way can result in immunological tolerance. While parenteral administration of protein can avoid the development of oral tolerance but can provide a clear indication of inherent ability of proteins to induce IgE antibody responses. Therefore, FAO/WHO recommended that the results from two sensitization routes should be considered. FAO/WHO also suggested that the potential allergenicity of the expressed protein be ranked against well-known strong and weak food allergens and non-allergenic proteins in the animal models.
For these reasons, in the present study, the allergenicity of different proteins were compared, including ovalbumin (OVA), a potent respiratory and food allergen, bovine serum albumin (BSA), a protein that is considered to have a lesser allergenic potential, and potato acid phosphatase (PAP), a non-allergenic protein when administered to BN rats intraperitoneally or by gavage.
Male BN rats, 4-6-wk old, were obtained from the Animal Center of the Chinese Academy of Medical Sciences (Beijing, China). The rats were housed in an animal room maintained at 24 ± 1°C and 50% ± 10% relative humidity with the altering 12:12-h light-dark cycle. The animals were housed in stainless-steel wire cages in groups of five and had free access to milk and egg-free diet and water.
OVA, a potent respiratory and food allergen, and BSA, a protein that is considered to have a lesser allergenic potential, were obtained from Sigma Chemicals, USA. PAP also purchased from Sigma was used as a non-allergenic protein.
Animals were randomly divided into three groups, 10 rats in each group. The rats were exposed by gavage to OVA, BSA, or PAP (1 mg protein/mL tap water; 1 mL/animal) respectively for 6 wk, without the use of an adjuvant. At weekly intervals, blood samples were obtained from the orbital plexus. Blood samples for IgG antibody and reagnic antibody analysis were centrifuged for 20 min at 2 000 r/min to obtain sera. Blood samples for plasma histamine level determination were collected into chilled tubes containing EDTA-K2 to obtain plasma aliquots.
Rats were randomly divided into three groups, 10 rats in each group. The animals were exposed intraperitoneally to OVA, BSA, or PAP (100 µg protein/mL tap water; 1 mL/animal) respectively on days 0 and 7. Animals were bled at weekly intervals from d 7 to 42. Blood samples for IgG antibody and reagnic antibody analysis were centrifuged for 20 min at 2 000 r/min to obtain sera. Blood samples for plasma histamine level determination were collected into chilled tubes containing EDTA-K2 to obtain plasma aliquots.
Antigen-specific IgG was determined using an ELISA technique. Solution of OVA, BSA, or PAP (10 µg/mL) was bound to a 96-well microtiter plates (100 µL/well) overnight at 4°C. The plates were washed thrice with tap water containing 0.5% Tween 20. This was followed by the addition of 100 µL/well PBS containing 0.5% Tween 20 and 2% sheep serum albumin. After 1-h incubation at 37°C, the plates were washed and serial dilutions of rat serum were added to the wells and incubated for 1 h at 37°C. After washing, 100 µL/well peroxidase conjugated goat anti-rat IgG (H+L) (Zymed, USA) was added. After incubation for 1 h at 37°C, the plates were washed again and an enzyme-substrate solution of tetramethylbenzidine (Sigma, 100 µL/well) was added. The plates were developed at room temperature for 10 min. Finally, 100 µL/well of 1 mol/L H2SO4 was added. Optical densities were read at 450 nm with an ELISA plate reader. A pre-serum pool was used as negative control. The pooled pre-serum was measured at a 1:4 dilution. The average extinction in negative control wells, to which three times the standard deviation was added, provided the reference value taken to determine the titer in the test sera. Each test serum was titrated starting at a 1:4 dilution, and the reciprocal of the greatest serum dilution giving an extinction higher than the reference value was read as the titer. The titer >25 was considered as positive IgG antibody responses.
The presence of reaginic antibody was assessed by passive cutaneous anaphylaxis assay (PCA)[7]. Naïve BN rats were shaved on the back and flanks and injected intradermally with 0.1 mL of the test sera in serial dilutions followed 64 h later with an intravenous injection of 1 mL of 1:1 mixture of a solution of OVA, BSA, or PAP (5 mg/mL) and a solution of Evans blue (2% in sterile saline). After 20-30 min, the animals were examined for positive responses. The diameter of dye extravasation at the site of the serum injection was measured. The positive response was considered when a colored spot of at least 5 mm in diameter appeared.
In order to evaluate the plasma histamine level, blood samples of rat were collected into chilled tubes containing 40 µL of 7.5% potassium ethylenediamine tetraacetic acid (EDTA-K2) on d 7 after antigen injection or antigen exposure by gavage. After centrifugation, plasma aliquots were collected and frozen at -80°C until use. Plasma histamine levels were determined using an immunoassay kit (IBL, Germany) following the manufacturer’s instructions.
Before challenge, individual baseline blood pressures were determined on two separate days. Prior to blood pressure measurements, the animals were placed under a heating light (30°C) for 20 min. Thereafter, an inflatable pressure cuff was put around the tail and a distal sensor was used to record the systolic blood pressure. The OVA, BSA, or PAP sensitized and control animals were challenged orally with 2 mL of a 5 mg/mL OVA, BSA, or PAP solution or 2 mL of tap water. Blood pressure was recorded at intervals for over a period of 7 h.
Plasma histamine levels were compared among three groups by Student’s t-test.
During the whole study of either exposure routes, OVA and BSA provoked protein-specific IgG antibody responses, while PAP did not elicit protein-specific IgG antibody responses (Tables 1 and 2).
| Groups | Number of responders on day | ||||
| 14 | 21 | 28 | 35 | 42 | |
| OVA | 10 | 10 | 10 | 10 | 10 |
| BSA | 5 | 7 | 8 | 8 | 6 |
| PAP | 0 | 0 | 0 | 0 | 0 |
| Groups | Number of responders on day | ||||
| 14 | 21 | 28 | 35 | 42 | |
| OVA | 10 | 10 | 10 | 10 | 10 |
| BSA | 6 | 8 | 8 | 8 | 7 |
| PAP | 0 | 0 | 0 | 0 | 0 |
Reaginic antibody responses determined by PCA were shown in Tables 3 and 4. Strong OVA-specific IgE antibody responses were provoked, only a limited BSA-specific IgE responders were observed and PAP did not elicit protein-specific IgE antibody responses.
| Groups | Number of responders on day | ||||
| 14 | 21 | 28 | 35 | 42 | |
| OVA | 8 | 8 | 9 | 9 | 8 |
| BSA | 2 | 3 | 3 | 3 | 2 |
| PAP | 0 | 0 | 0 | 0 | 0 |
| Groups | Number of responders on day | ||||
| 14 | 21 | 28 | 35 | 42 | |
| OVA | 8 | 9 | 9 | 9 | 8 |
| BSA | 3 | 4 | 4 | 4 | 3 |
| PAP | 0 | 0 | 0 | 0 | 0 |
In either routes of exposure, plasma histamine level in BN rats sensitized with OVA was higher than that of BSA or PAP (Tables 5 and 6).
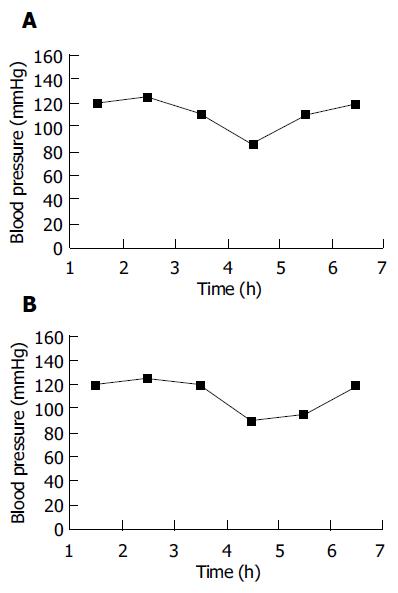
An oral challenge with BSA and PAP did not induce any effect on blood pressure, while a temporary drop in systolic blood pressure in three animals of each routes of exposure was found by an oral challenge with OVA. Representative results of blood pressure changes induced by OVA via oral sensitization or intraperitoneal sensitization are shown in Figure 1.
Food allergy is an important health issue of growing interest. By definition, food allergy is an adverse reaction to a harmless food or food components that involves an abnormal response of the body’s immune system to specific protein in foods. The most common type of food allergy is mediated by allergen-specific IgE[10]. Sensitization is induced by following exposure of the susceptible individual to the protein allergen sufficient to stimulate an IgE antibody response. If the now-sensitized individual is exposed subsequently to the same protein, then antigen cross-links specific membrane-bound IgE antibodies and this in turn causes mast cell degranulation and the release of inflammatory mediators such as histamine, cytokine, and so on, which together initiate the symptoms of food allergy[11,12].
Not all proteins display allergenic potential, despite being immunogenic (able to stimulate IgG antibody responses), proteins appear to differ markedly with respect to their ability to cause IgE-mediated allergic sensitization[13]. Therefore, in this study, IgG antibody response (antigen), IgE antibody response (allergen), histamine level (inflammatory mediators) and blood pressure (systemic challenge effects) were used as parameters to develop the BN rat model.
Collectively, our study has shown that BN rats, bred and raised on a diet free of the antigen to be tested, can be sensitized by daily dosing with the antigen via enteral route or intraperitoneal injection without use of adjuvants. However, there appears to be significant differences between the proteins examined with respect to IgE antibody response. By either route of exposure, OVA provoked very vigorous protein-specific IgG and IgE responses, low grade protein-specific IgG and IgE responses were elicited by BSA, while by neither route did PAP elicit anything. The same phenomenon was described in BN rats sensitized by daily intra-gastric administration of OVA, hen’s egg white (HEW) and cow’s milk (CM) proteins[14]. In that study, OVA provoked strong antigen-specific IgG as well as IgE responses in almost all rats, while only a limited number of IgE responders were observed in rats with HEW or CM. These results indicate that BN rats demonstrate IgE antibody responses to a comparable selection of proteins upon exposure of different proteins and support that the BN rat may provide a suitable animal model for assessing the allergenicity of novel food proteins and for research on the mechanisms of food allergy. For a more detailed characterization of the rat model developed, additional studies were performed to study the release of inflammatory mediators (histamine) and systemic immune-mediated effects (blood pressure).
Histamine is a potent mediator of numerous biological reactions. In the human organism, it is virtually ubiquitous in tissues and body fluids, being mainly stored in its inactive form in the metachromatic granula of mast cells and basophilic leucocytes. On release, histamine functions as a potent mediator of numerous physiological and pathophysiological processes in nearly all organs and tissues[11,12]. Histamine has been clearly implicated as a primary mediator of “immediate type” allergic reactions (IgE-mediated allergic sensitization). In this study, the plasma histamine level in rats sensitized by OVA was higher than that of BSA and PAP, which is consistent with the results of antigen-specific IgE responses. Also, other mediators such as platelet-activating factor, prostaglandins, leukotrienes and some newly formed cytokines release in anaphylactic reactions have been studied in experimental models.
The possible systemic effect was investigated by monitoring blood pressure. An oral challenge with BSA and PAP did not induce any effect on blood pressure, while a temporary drop in systolic blood pressure in some animals was found by an oral challenge with OVA. Although this effect was observed in only a few animals (around 30%), this low incidence is in agreement with observations from food allergic patients. The results indicate that systemic effects can be induced upon an oral challenge with allergens such as OVA.
In conclusion, although additional studies are needed with more purified strong and weak allergens, non-allergens, and allergenic whole foods, to further validate the developed BN rat model, the results obtained in this study indicate that BN rat model might be a useful and predictive animal model to study the potential allergenicity of novel food proteins.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Metcalfe DD, Astwood JD, Townsend R, Sampson HA, Taylor SL, Fuchs RL. Assessment of the allergenic potential of foods derived from genetically engineered crop plants. Crit Rev Food Sci Nutr. 1996;36 Suppl:S165-S186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 252] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Kimber I, Betts CJ, Dearman RJ. Assessment of the allergenic potential of proteins. Toxicol Lett. 2003;140-141:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Taylor SL, Lehrer SB. Principles and characteristics of food allergens. Crit Rev Food Sci Nutr. 1996;36 Suppl:S91-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Dearman RJ, Kimber I. Determination of protein allergenicity: studies in mice. Toxicol Lett. 2001;120:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Piacentini GL, Vicentini L, Bodini A, Mazzi P, Peroni DG, Maffeis C, Boner AL. Allergenicity of a hydrolyzed rice infant formula in a guinea pig model. Ann Allergy Asthma Immunol. 2003;91:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Knippels LM, Penninks AH, Spanhaak S, Houben GF. Oral sensitization to food proteins: a Brown Norway rat model. Clin Exp Allergy. 1998;28:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Penninks AH, Knippels LM. Determination of protein allergenicity: studies in rats. Toxicol Lett. 2001;120:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Knippels LM, Penninks AH, Smit JJ, Houben GF. Immune-mediated effects upon oral challenge of ovalbumin-sensitized Brown Norway rats: further characterization of a rat food allergy model. Toxicol Appl Pharmacol. 1999;156:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Knippels LM, Houben GF, Spanhaak S, Penninks AH. An oral sensitization model in Brown Norway rats to screen for potential allergenicity of food proteins. Methods. 1999;19:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Taylor SL, Hefle SL. Will genetically modified foods be allergenic? J Allergy Clin Immunol. 2001;107:765-771. [PubMed] |
| 11. | Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004;34:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol. 2005;137:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Kimber I, Kerkvliet NI, Taylor SL, Astwood JD, Sarlo K, Dearman RJ. Toxicology of protein allergenicity: prediction and characterization. Toxicol Sci. 1999;48:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Knippels LM, van der Kleij HP, Koppelman SJ, Houben GF, Penninks AH. Comparison of antibody responses to hen's egg and cow's milk proteins in orally sensitized rats and food-allergic patients. Allergy. 2000;55:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |