Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5373
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: September 14, 2005
AIM: To explore the effect of Gui Zhi decoction on enteric mucosal immune in type II collagen-induced arthritis (CIA) in DBA mice.
METHODS: Eighty DBA/1, weighing 18-22 g, were randomly divided into four groups with 20 in each group: control group, CIA group, treatment groups at high dosage and low dosage (GZH and GZL). CIA was induced by immunization with type II collagen (CII) emulsified with equal complete adjuvant at 0.1 mg CII each mouse. Blood lymphocyte suspension was screened for CD4 and CD8 expression using a flow cytometry, the CD4 and CD8 and secretory IgA (sIgA)-positive cells in enteric lamina propria tested with immunohistochemical staining. Tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1)-β, and IL-6 concentrations in serum were assayed with RIA.
RESULTS: Gui Zhi decoction can lower the arthritic scores and decrease the occurrence of arthritis. The CD4, CD8, and sIgA-positive cells in CIA mice are less than in control mice, and in Gui Zhi decoction at high dosage could restore the lowered CD4- and CD8-positive cells in lamina propria, and at both high and low dosages could increase the lowered sIgA-positive cells in lamina propria, even still lower than in normal mice. In periphery, the CD4 cells in periphery are higher in CIA mice than in control mice, and Gui Zhi decoction at high and low dosages could decrease the CD4 and CD8 cells. Also, Gui Zhi decoction at high dosage could decrease the IL-6 and TNF-α concentration in serum.
CONCLUSION: Gui Zhi decoction can lower the arthritic scores and decrease the incidence of CIA in mice, and the mechanism is in part regulating enteric mucosal immune.
- Citation: Zhou GQ, Zhao N, Zhang H, Jia HW, Zhang WD, Zhao LH, Lu C, He YH, Lu AP. Effect of Gui Zhi decoction on enteric mucosal immune in mice with collagen-induced arthritis. World J Gastroenterol 2005; 11(34): 5373-5376
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5373
Mucosal immune system is an integrated network of tissues, lymphoid and constitutive cells and effector molecules characterized by production of secretory IgA (sIgA), Th1-and Th2-type CD4 T-lymphocytes responses and CD8 CTL responses. It functions through protecting the host from infection of the mucous membrane surfaces, on the other hand, it can induct tolerance to the antigen in the food, and now it has become the focus of more and more scientists[1,2].
Gui Zhi decoction is a classical herbal preparation, and functions as regulating Ying and Wei in traditional Chinese medicine. It is commonly used as one of the therapies of rheumatic arthritis and rheumatoid arthritis[3]. While how the Gui Zhi decoction works in anti-rheumatic effect and how the enteric mucosal immune is involved in the mechanism is less clarified.
Collagen-induced arthritis (CIA) is a common animal model for auto-immune disease, special for the research on rheumatoid arthritis, since its pathology and immunology is similar to that in human rheumatoid arthritis[4]. In this paper, the effect of Gui Zhi decoction on enteric immune in CIA mice was explored.
Total 80 DBA/1 mice, male, 8-10-wk old with a mean weight of 18-22 g were purchased from Research Institute of Experimental Animals, Chinese Academy of Medical Science. Mice were randomly divided into four groups in average: control group, CIA group, treatment groups at high dosage and low dosage (GZH and GZL). Mice were housed in a temperature-, humidity- and light-controlled environment with free access to rodent chow and water. The light-dark cycle was 12:12 h with the light phase from 06:00 a.m. to 18:00 p.m. The rodent license of the laboratory (No. SCXK11-00-0006) was issued by National Science and Technology Ministry of China.
Soluble pure type II collagen (CII) was from Dr. Rikard Holmdahl (Lund University, Sweden) and complete adjuvant was purchased from Sigma Corporation, USA. CIA was induced by II collagen, dissolved in 0.1 mol/L acetic acid (2 mg in 1 mL) emulsified with equal complete adjuvant and 0.1 mol/L acetic acid. Each rat in CIA group and treatment group was immunized by injection of mixture (containing 0.15 mg CII) under fur skin at the left sole of the foot. Starting from 14th d after immunization, the degree of arthritis was examined every 2 d. The severity of arthritis was represented as mean arthritic index on a 0-4 scale according to the following criteria: 0 = no edema or swelling; 1 = slight edema and erythema limited to the foot and/or ankle; 2 = slight edema and erythema from the ankle to the tarsal bone; 3 = moderate edema and erythema from the ankle to the tarsal bone; and 4 = edema and erythema from the ankle to the entire leg. Each limb was graded, and thus the maximum possible score was 16 for each animal. A rat or mice with a score of 1 or more was regarded as arthritic.
Gui Zhi decoction recipe was a classical one, and consists of Ramulus Cinnamomi, Radix Paeoniae Alba, Rhizoma Zingiberis Recens, and Fructus Jujubae at the ratio of 10: 10:7:10:10. The herbal mixtures were extracted by boiling water to the concentration at 1 g/mL. The dosage in GZH was at 30 g/kg (body weight) and in GZL at 8.75 g/kg. All the agents were orally administered in a volume of 0.8 mL/mouse. The animals in normal group and CIA group were administered with the same volume of saline. The admini-strations were conducted that started from 2 wk after the immunization, once a day and lasted for 4 wk.
The CD4-, CD8- and sIgA-positive cells in enteric lamina propria were tested by immunohistochemical staining method with the test kits. The staining process referred to the manuals for the products. Briefly, on d 36, animals were euthanized under anesthesia, and the mid-section of small intestine were removed and fixed in 10% paraformaline, embedded in paraffin, sectioned at 5 µm, then ice acetone fixed for 10 min. The first antibodies were rabbit anti-rat antibodies, and the second antibodies were goat anti-rabbit antibodies, and the samples were stained with DAB (Sigma, USA) and counterstained with hematoxylin. The positive granules can be observed. The positive granule averages of three samples (6 scopes in each sample) from Q-win DC100 image analysis were used for further statistical analysis.
The blood was sampled before the animals were killed, and suspended in tubes containing 25 µL heparin (5 000 IU/mL). The erythrocytes were lyzed in a buffer containing 0.84% NH4Cl at pH 7.4 for 3-5 min. The remaining cells were then washed twice in saline. After washing in saline, the cells were resuspended and fixed in 500 µL saline. Blood lymphocyte suspension was screened for CD4 and CD8 expression using a flow cytometry. The mAbs used for CD4 staining were R-Phycoerythrin-conjugated OX-38 (BD Biosciences Pharmingen) and CD8a staining were Peridinin Chlorophyll-a Protein-conjugated OX-8 (BD Biosciences Pharmingen). Each sample of 0.1 mL peripheral lymphocyte or PP single-cell suspension was incubated for 30 min at 4°C in the dark, with a solution consisting of 6 µL 0.01 mol/L PBS and an appropriate concentration of antibodies. After washing in saline, the cells were resuspended and fixed in 500 µL saline. The cells were then analyzed on a FACSort using the CellQuest software (Becton-Dickinson).
Tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6 concentrations in serum were assayed using mouse cytokine RIA test kits (Wuhan Boster Biotech Inc., China). The assay was performed in duplicate according to the manufacturer’s recommended procedures. The results of the radioactivity count were recorded by an automatic Gamma counter (SN-695B), and expressed as mean±SE of individual rat.
Using SPSS 10.0 software, ANOVA was used to determine significance in the data set. Student-Newman-Keuls test was employed for variables between both groups when equal variances assumed and Dunnett’s t-test for equal variances not assumed.
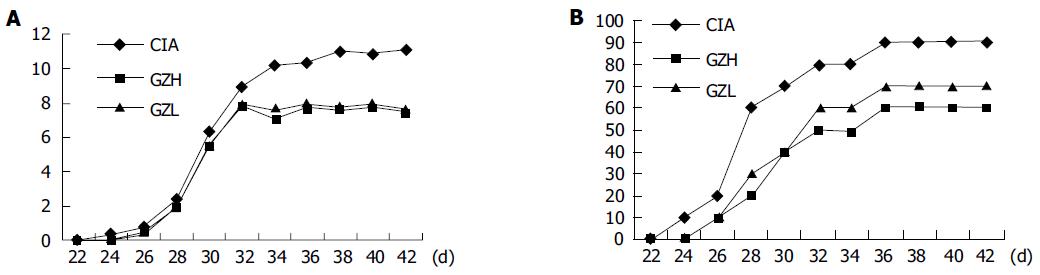
Figure 1A shows the changes of arthritic scores in CIA group and Gui Zhi decoction-treated groups. Figure 1B shows the changes of arthritis incidence, and there is a significant difference in CIA group and Gui Zhi decoction-treated groups. These results demonstrate that Gui Zhi decoction can significantly lower the arthritic scores and decrease the occurrence of arthritis.
The positive expression of CD4, CD8, and sIgA were shown as brown granules in the sections. Most of CD4-positive lymphocytes and sIgA-positive cells were located in lamina propria, and majority of CD8-positive lymphocytes were located in epithelial surfaces. Table 1 shows the positive-stained granules from six scopes under microscope, which represents the numbers of CD4- and CD8-positive cells. The results demonstrate that the CD4- and CD8-positive cells in lamina propria in CIA mice are less than in control mice, and in Gui Zhi-decoction-treated mice at high dosage and they are similar to that in the control mice. The results suggest that the enteric mucosal immunity to some extent was inhibited in CIA mice, and Gui Zhi decoction at high dosage could recover the mucosal immunity.
Table 2 shows that the sIgA-positive cells in lamina propria in CIA mice are less than in control mice, and in Gui Zhi-decoction-treated mice they are similar to that in the control mice, but still lower than in control mice.
The results suggest that the enteric mucosal immunity was inhibited in CIA mice by showing decrease of the CD4 and CD8 cells in enteric lamina propria, and Gui Zhi decoction could recover to some extent the CD4-, CD8-and sIgA-positive cells in lamina propria, and further might recover the inhibition role of mucosal immunity.
Table 3 shows that the CD4 cells in periphery are higher in CIA mice than in control mice, the CD8 cells in CIA mice are similar to those in control mice. In the mice treated with Gui Zhi decoction at high and low dosages, CD4 and CD8 cells decrease.
Table 4 shows that the IL-6, IL-1β, and TNF-α concentrations are higher in CIA mice than in control mice, and after being treated with Gui Zhi decoction at high dosage, the IL-6 and TNF-α concentration decrease comparing to the concentrations in CIA mice.
Lymphoid tissue in the human body is associated with the mucosal system. Mucosa-associated lymphoid tissue includes gut-associated lymphoid tissue, bronchial/tracheal-associated lymphoid tissue, nose-associated lymphoid tissue, and vulvovaginal-associated lymphoid tissue, as the first barrier of the human body, protects the body from antigens entering along mucosal surfaces[5]. Functional disturbance of mucosal system may lead to autoimmune diseases or downregulation of immune function[6,7], which has some similarity with the function of “Ying and Wei” in traditional Chinese medicine.
The gastrointestinal mucosa is a vast interface between the body and the environment; it is the main entry site for many environmental antigens. The influx of multiple antigens through the gastrointestinal mucosa usually results in tolerance. High-dose tolerance is due to T cell deletion or anergy, whereas low-dose tolerance involves activation of TGF-β producing Th2 or Th3 cells. This intolerance effect has been used to try to induce oral tolerance. For instance, pre-treatment with oral bovine type II collagen has proved capable of modulating several models of experimental polyarthritis. Arthritis severity was considerably reduced[8]. Nasal admin istration of 10 μg of CII 15 times had the most prominent suppressive effects, reducing disease incidence by 50% and inhibiting both CII-specific IgG antibody and DTH responses[9].
The enteric immune cells could be considered to be the effector cells in regarding to deal with food antigen, and also would be the inducer cells with regard to tolerance induction. However, what about the change of enteric immune in autoimmune is not well known. Our group reported a primary result on how Gui Zhi decoction affects on the numbers of enteric mucosal lamina propria CD4-, CD8-and sIgA-positive cells[10,11], and the results suggest that the enteric mucosal immune might involve the pathway of CIA in mice.
In this paper, the results show that Gui Zhi decoction can significantly lower the arthritic scores and decrease the occurrence of arthritis. The CD4 cells in periphery are higher in CIA mice than in control mice, and the results are similar to the previous study. In the mice treated with Gui Zhi decoction at high and low dosages, CD4 and CD8 cells in periphery decrease. The CD4- and CD8-positive cells in enteric lamina propria in CIA mice are less than in control mice, and in Gui Zhi-decoction-treated mice at high dosage they are similar to that in the control mice. The sIgA-positive cells in lamina propria in CIA mice are less than in control mice, and in Gui Zhi-decoction-treated mice they are similar to that in the control mice, but still lower than in control mice, suggesting that the enteric mucosal immunity to some extent was inhibited in CIA mice. The results suggest that the enteric mucosal immunity was inhibited in CIA mice by showing the decrease of the CD4 and CD8 cells in enteric lamina propria, and Gui Zhi decoction could recover to some extent the CD4-, CD8- and sIgA-positive cells in lamina propria, and further might recover the inhibition role of mucosal immunity.
The onset of arthritis in IL-6-/- mice was delayed for 2 wk compared with that in IL-6+/+ mice, and the severity of arthritis, as indicated by the arthritis score, remained significantly lower in IL-6-/- mice during the entire follow-up period (14 wk), although all IL-6-/- mice developed definite arthritis as did the IL-6+/+ mice. These findings suggest that blockade of IL-6 might be beneficial in the treatment of RA[12-14]. IL-1 and TNF-α play dominant roles in mediating the progression of many inflammatory joint diseases, including rheumatoid arthritis in humans, CIA in mice and rats, and adjuvant arthritis in rats. Treating autoimmune arthritic diseases with combinations of anti-IL-1 and anti-TNF molecules will achieve superior efficacy compared to the use of a single class of anti-cytokine agent and may allow for dose reductions that could prove useful in minimizing potential side effects[15]. In this paper, the results show that the IL-6, IL-1β, and TNF-α concentrations are higher in CIA mice than in control mice, and after being treated with Gui Zhi decoction at high dosage, the IL-6 and TNF-α concentration decrease comparing to the concentrations in CIA mice.
In conclusion, Gui Zhi decoction could lower the arthritic scores, and regulate the enteric mucosal immune, and the results indicate that the enteric mucosal immune might involve in the pathogenesis of CIA, and also it might be an important target for anti-rheumatic therapy. However, more detailed studies on how enteric mucosal immune are involved in the pathogenesis of CIA, and how the mucosal immune serves as a pathway for the treatment of CIA need further research works.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Didierlaurent A, Sirard JC, Kraehenbuhl JP, Neutra MR. How the gut senses its content. Cell Microbiol. 2002;4:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Tlaskalová-Hogenová H, Tucková L, Lodinová-Zádniková R, Stepánková R, Cukrowska B, Funda DP, Striz I, Kozáková H, Trebichavský I, Sokol D. Mucosal immunity: its role in defense and allergy. Int Arch Allergy Immunol. 2002;128:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Lu XF. [Experimental study on the immunosuppressive effects of gui zhi tang]. Zhong Xi Yi Jie He Za Zhi. 1989;9:283-25, 262. [PubMed] |
| 4. | Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 5. | Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 342] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Yuan Q, Walker WA. Innate immunity of the gut: mucosal defense in health and disease. J Pediatr Gastroenterol Nutr. 2004;38:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Tishler M, Caspi D, Almog Y, Segal R, Yaron M. Increased incidence of urinary tract infection in patients with rheumatoid arthritis and secondary Sjögren's syndrome. Ann Rheum Dis. 1992;51:604-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Kim WU, Lee WK, Ryoo JW, Kim SH, Kim J, Youn J, Min SY, Bae EY, Hwang SY, Park SH. Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002;46:1109-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Higuchi K, Kweon MN, Fujihashi K, McGhee JR, Kiyono H. Comparison of nasal and oral tolerance for the prevention of collagen induced murine arthritis. J Rheumatol. 2000;27:1038-1044. [PubMed] |
| 10. | Zhou GQ, Xiao C, Zhou J. Effect of guizhi decoction on CD4+, CD8+ T-lymphocytes and SIgA in mucosal immune system in intestine of mice with Bi syndrome (collagen induced immune arthritis). Zhongguo Zhongxiyi Jiehe ZaZhi. 2004;24:336-338. [PubMed] |
| 11. | Heel KA, McCauley RD, Papadimitriou JM, Hall JC. Review: Peyer's patches. J Gastroenterol Hepatol. 1997;12:122-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Sasai M, Saeki Y, Ohshima S, Nishioka K, Mima T, Tanaka T, Katada Y, Yoshizaki K, Suemura M, Kishimoto T. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Feige U, Hu YL, Gasser J, Campagnuolo G, Munyakazi L, Bolon B. Anti-interleukin-1 and anti-tumor necrosis factor-alpha synergistically inhibit adjuvant arthritis in Lewis rats. Cell Mol Life Sci. 2000;57:1457-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Beagley KW, Eldridge JH, Kiyono H, Everson MP, Koopman WJ, Honjo T, McGhee JR. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J Immunol. 1988;141:2035-2042. [PubMed] |
| 15. | Beagley KW, Eldridge JH, Lee F, Kiyono H, Everson MP, Koopman WJ, Hirano T, Kishimoto T, McGhee JR. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133-2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 349] [Article Influence: 9.7] [Reference Citation Analysis (0)] |