Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5358
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: September 14, 2005
AIM: To study the effects of indomethacin on the isolated transverse and longitudinal rat gastric fundus strips.
METHODS: The strips were suspended in an organ bath containing oxygenated Krebs solution, and contractile responses to electrical field stimulation were recorded on a physiograph in an isotonic manner after administration of cumulative concentrations of indomethacin. The effects of indomethacin on the strips pretreated with KATP channel modulators, diazoxide and glybenclamide were studied.
RESULTS: Treatment of the transverse strips with indomethacin resulted in a concentration-dependent inhibitory response. In longitudinal strips, biphasic responses were seen, which included a stimulatory response at low concentrations of indomethacin, followed by an inhibitory response at higher concentrations. Diazoxide pre-treatment inhibited the stimulatory response of longitudinal strips. Glybenclamide pre-treatment not only blocked inhibitory effect of the low concentrations of indomethacin on transverse strips, but also increased the amplitude of contractions. Moreover, the drug decreased the amplitude of contractions in longitudinal strips.
CONCLUSION: Responses of the isolated longitudinal and transverse rat gastric fundus strips to indomethacin are not similar, and are influenced by KATP channel modulators.
- Citation: Afshin S, Keshavarz M, Salami M, Mirershadi F, Djahanguiri B. Effect of indomethacin on electrical field stimulation-induced contractions of isolated transverse and longitudinal rat gastric fundus strips. World J Gastroenterol 2005; 11(34): 5358-5361
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5358
Hypermotility of the stomach is proposed to be one of the mechanisms of the ulcerogenic action of indomethacin in rats[1-3]. It has been suggested that the stomach hypermotility plays a key role in the onset of the lesions, and the other factors are involved in the later extension of damage induced by indomethacin[2,4-6]. Ueki et al[3] demonstrated that administration of atropine and 16,16-dimethyl PGE2 suppressed gastric hypermotility and reduced gastric ulcerations. On the other hand, the KATP channels have also been shown to play a part in the pathogenesis of indomethacin-induced gastric ulceration[7-10].
In the present work, since our preliminary studies showed that indomethacin has relaxatory effects on the strips, we have studied the effects of different concentrations of indomethacin on contractile response of the isolated longitudinal and transverse rat gastric fundus strips stimulated by the electrical field stimulation (EFS). The effects of diazoxide as KATP channel opener and glybenclamide as the channel antagonist on the contractile pattern of the stomach were studied as well.
Male Sprague-Dawley rats (160-200 g) were used. They were fasted overnight and killed by a blow on the head, followed by removal of the stomach through abdominal incision. Longitudinal and transverse strips of the gastric fundus were prepared as previously described by Milenov and Kalfin[11]. The strips were 4-5 mm wide and 15-20 mm long. Not more than four strips were prepared from each stomach. The strips were suspended under 1 g preload in a 20 mL organ bath containing oxygenated Krebs solution (113 mmol/L NaCl, 2.5 mmol/L CaCl2, 4.7 mmol/L KCl, 25 mmol/L NaHCO3, 1.1 mmol/L MgSO4, 1.1 mmol/L KH2PO4, and 11 mmol/L glucose at the pH of 7.2). Pre-warmed water (37°C) was circulated through the outer jacket of the tissue bath via constant-temperature circulator pump. The temperature of the Krebs solution in the organ bath was maintained within 37 ± 0.5°C. The strips were allowed to equilibrate for approximately 45 min by the change of bath solution every 15 min. After stabilization of the tissue, EFS was applied via two platinum electrodes through which the preparation was surrounded by. Electrical impulses (rectangular waves, 50 V, 20 Hz, 1 ms for 8 s train duration with 100-s intervals between the trains) were provided by a modified stimulator (Harvard 6002, USA). The stimulus resulted in uniform strip contractions which were recorded via isotonic transducer and Universal Oscillograph (Harvard Apparatus, USA).
Cumulative concentrations of indomethacin (3 × 10-7 -2 × 10-4 mol/L) were added to the organ bath for evaluation of effects of indomethacin. In a series of experiments, the strips were pretreated with diazoxide (10-4 mol/L) or glybenclamide (4 × 10-6 mol/L) followed, 15 min later, by the administration of cumulative concentrations of indomethacin.
All drugs were purchased as pure powder except diazoxide (aqueous solution). Tween 20 was used for the preparation of drug suspensions (indomethacin and glybenclamide). Drug concentrations were adjusted so that the volume of the added drug to the organ bath exceeds not more than 0.2 mL.
Each tissue was used for one dose-response curve. This was repeated with eight different tissues. The contractile response of each intact tissue to the EFS was considered as control (100%), and amplitude of the responses to different concentrations of drugs were compared with it. The means and SE for response to each concentration were calculated. Student’s t-test and two-way analysis of variance with Newman-Keuls post hoc were used to test the statistical significance between different groups. Data were expressed as mean ± SE. P<0.05 was considered statistically significant.
EFS produced uniform contractile responses in both longitudinal and transverse strips, which lasted for more than 100 min and were abolished following atropine treatment. Vehicle had no effect on contractions.
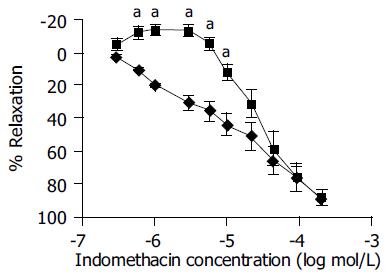
In the transverse strips, administration of cumulative concentrations of indomethacin (3 × 10-7-2 × 10-4 mol/L) produced concentration-dependent decrease in amplitude of contractile response. The inhibitory effect of indomethacin started from the concentration of 3 × 10-7 mol/L and its maximum effect was obtained at the concentration of 2 × 10-4 mol/L. In the longitudinal strips, indomethacin, at low concentrations (3 × 10-7-6 × 10-6 mol/L), increased the amplitude of contractile responses. The peak of increase was 14.1 ± 3.14%. With higher concentrations (10-5-2 × 10-4 mol/L), a decrease in contractile response was observed (Figure 1).
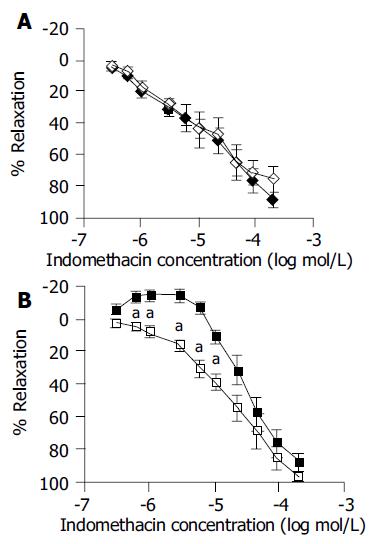
Pretreatment with diazoxide (10-4 mol/L), a KATP channel opener, 15 min before indomethacin administration showed no change in the response to indomethacin of transverse strip (Figure 2A). However, in longitudinal strips, “primary contractile response” was abolished and a continuous relaxation was seen following the administration of indomethacin. The concentration-response curve was shifted to the left and down and became similar to the transverse strip responses to indomethacin alone (Figure 2B).
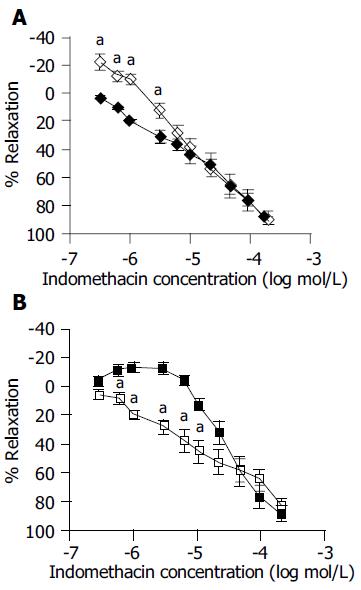
Glybenclamide pre-treatment (4 × 10-6 mol/L) 15 min before indomethacin administration, not only prevented “acute inhibitory response” in transverse strips, but also increased the amplitude of contractile responses to indomethacin at concentrations of 3 × 10-7-10-6 mol/L. The curve shifted to the right and up and became similar to longitudinal strip response to indomethacin alone (Figure 3A). Longitudinal strip responses to indomethacin decreased in the presence of glybenclamide, resulting in a shift to the left and down of the response curve and making the curve similar to the response curve of transverse strip to indomethacin alone (Figure 3B).
Several mechanisms, including the hypermotility of the stomach, have been proposed to explain indomethacin-induced gastric ulceration[1-4]. Hypermotility may be due to the action of drugs on the CNS, or peripheral effects of indomethacin on the stomach nerves or muscles. In the present study, we have investigated the effects of indomethacin on the contractile responses of the isolated transverse and longitudinal strips of rat gastric fundus to myenteric plexus activation via EFS.
The fact that atropine prevented the EFS-induced strip contractions suggests that the contractions originate, at least in part, from the cholinergic system. According to the present results, the responses of transverse and longitudinal strips to indomethacin were not identical. In the transverse strips, the effect of indomethacin was inhibitory while in the longitudinal strips, the response to indomethacin was dependent on the concentrations of the drug. We observed an increase in the amplitude at the low concentrations, while a decrease in the amplitude at higher concentrations. Takeuchi et al[1,2] and Ueki et al[3] in in vivo studies, demonstrated that indomethacin administration resulted in a significant gastric hypermotility. In our experiments, contrary to the hypermotility reported by the aforementioned investigators, we observed relaxation of the strips after indomethacin administration. The reason for this discrepancy may be due to the fact that in the previous published reports, the effects of indomethacin have been studied in the intact animals (in vivo) while the present work is an in vitro study, independent of humoral and/or neural influences. It is possible that the reported results are the net effect of several different factors; some of them are not limited to the stomach and are systemic effects. Gastric motility is under the control of neural, humoral, and mechanical factors with stimulatory or inhibitory effects on the stomach contractility. The net results of these effects will determine the contractility of the stomach. The strip relaxation may be due to the inhibition of stimulatory prostaglandins (PGs)[12,13], L-type calcium channels[14] or phosphodiesterase 4[15].
The presence of the KATP channels has been demonstrated in the smooth muscles of the stomach[16]. The difference between the results obtained in transverse and longitudinal strips following indomethacin administration may be dependent on the state of KATP channels of the tissues. Opening of the channels by diazoxide, in the longitudinal strips, resulted in responses similar to that of transverse strips under indomethacin treatment. On the other hand, the administration of glybenclamide, a KATP channel antagonist, interestingly, changed the response of transverse strip to a response similar to that of longitudinal strips under indomethacin treatment. Altogether, these results are suggestive of the possible role played by KATP channels on the effects of indomethacin on the motility of the isolated rat gastric fundus strips.
The stomach is one of the sources of different types of PGs with stimulatory or inhibitory effects on contractility of the organ[17]. It has been known that indomethacin has inhibitory effects on the generation of PGs. On the other hand, the effects of PGs are known to be through their receptors[18]. Some PG receptors are only present in the longitudinal muscles[19]. This also may explain the difference between the results obtained in longitudinal and transverse strips as observed in our present study.
As shown in Figure 3, the stimulatory effect of indomethacin on the EFS-induced contraction of the longitudinal strip was abolished by both diazoxide and glybenclamide. These drugs have an opposing effect on KATP channels: diazoxide has an ability to open the channels while glybenclamide has an ability to inhibit the channels. Because KATP channel opener induces relaxation of smooth muscle through hyperpolarization, it is easy to understand the effect of diazoxide. However, glybenclamide causes depolarization through inhibition of KATP channels and results in activation of the cell (contraction). Yet, this agent abolished the stimulatory effect of indomethacin on the EFS-induced contraction of the longitudinal smooth muscle. It seems that glybenclamide acts more than a KATP channel blocker, thus other possible mechanisms for this action of glybenclamide must be evaluated.
In conclusion, the pattern of response of transverse and longitudinal strips to indomethacin is not quite similar, which may be due to the involvement of KATP channels. However, the overall effect of indomethacin on EFS-induced contractions of the isolated strips of rat gastric fundus is an inhibitory action.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Takeuchi K, Ueki S, Okabe S. Importance of gastric motility in the pathogenesis of indomethacin-induced gastric lesions in rats. Dig Dis Sci. 1986;31:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Takeuchi K, Tanaka A, Hayashi Y, Kubo Y. Functional mechanism underlying COX-2 expression following administration of indomethacin in rat stomachs: importance of gastric hypermotility. Dig Dis Sci. 2004;49:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Ueki S, Takeuchi K, Okabe S. Gastric motility is an important factor in the pathogenesis of indomethacin-induced gastric mucosal lesions in rats. Dig Dis Sci. 1988;33:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Takeuchi K, Kato S, Nishiwaki H, Hirata T. Analysis of pathogenic elements involved in gastric lesions induced by non-steroidal anti-inflammatory drugs in rats. J Gastroenterol Hepatol. 1997;12:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Suzuki K, Araki H, Komoike Y, Takeuchi K. Permissive role of neutrophils in pathogenesis of indomethacin-induced gastric lesions in rats. Med Sci Monit. 2000;6:908-914. [PubMed] |
| 6. | Okada M, Niida H, Takeuchi K, Okabe S. Role of prostaglandin deficiency in pathogenetic mechanism of gastric lesions induced by indomethacin in rats. Dig Dis Sci. 1989;34:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Toroudi HP, Rahgozar M, Bakhtiarian A, Djahanguiri B. Potassium channel modulators and indomethacin-induced gastric ulceration in rats. Scand J Gastroenterol. 1999;34:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Rahgozar M, Pazokitoroudi H, Bakhtiarian A, Djahanguiri B. Diazoxide, a K(ATP) opener, accelerates restitution of ethanol or indomethacin-induced gastric ulceration in rats independent of polyamines. J Gastroenterol Hepatol. 2001;16:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Akar F, Uydeş-Doğan BS, Buharalioğlu CK, Abban G, Heinemann A, Holzer P, Van de Voorde J. Protective effect of cromakalim and diazoxide, and proulcerogenic effect of glibenclamide on indomethacin-induced gastric injury. Eur J Pharmacol. 1999;374:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Peskar BM, Ehrlich K, Peskar BA. Role of ATP-sensitive potassium channels in prostaglandin-mediated gastroprotection in the rat. J Pharmacol Exp Ther. 2002;301:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Milenov K, Kalfin R. Cholinergic-nitrergic interactions in the guinea-pig gastric fundus. Neuropeptides. 1996;30:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Sanders KM, Northrup TE. Prostaglandin synthesis by microsomes of circular and longitudinal gastrointestinal muscles. Am J Physiol. 1983;244:G442-G448. [PubMed] |
| 13. | Sanders KM. Role of prostaglandins in regulating gastric motility. Am J Physiol. 1984;247:G117-G126. [PubMed] |
| 14. | Northover BJ. Indomethacin--a calcium antagonist. Gen Pharmacol. 1977;8:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Newcombe DS, Thanassi NM, Ciosek CP. Cartilage cyclic nucleotide phosphodiesterase: inhibition by anti-inflammatory agents. Life Sci. 1974;14:505-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Vogalis F. Potassium channels in gastrointestinal smooth muscle. J Auton Pharmacol. 2000;20:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Atay S, Tarnawski AS, Dubois A. Eicosanoids and the stomach. Prostaglandins Other Lipid Mediat. 2000;61:105-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193-1226. [PubMed] |
| 19. | Northey A, Denis D, Cirino M, Metters KM, Nantel F. Cellular distribution of prostanoid EP receptors mRNA in the rat gastrointestinal tract. Prostaglandins Other Lipid Mediat. 2000;62:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |