Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5351
Revised: January 10, 2005
Accepted: January 15, 2005
Published online: September 14, 2005
AIM: To investigate the possible relationships between gastric autoimmune phenomena and clinical presentations of this disorder, in consecutive atrophic body gastritis patients.
METHODS: A total of 140 atrophic body gastritis patients, diagnosed as consecutive outpatients presenting with macrocytic or iron deficiency anemia, or longstanding dyspepsia underwent gastroscopy with antral and body biopsies, assay of intrinsic factor, parietal cells and Helicobacter pylori (H pylori) antibodies. Gastritis was assessed according to Sydney System.
RESULTS: Parietal cell antibodies were equally distributed in all clinical presentations, whereas the positivity of intrinsic factor antibodies (49/140, 35%) was significantly higher in pernicious anemia patients (49.2%) than in iron deficiency (21.1%) and dyspeptic patients (27.8%). No specific pattern of autoantibodies was related to the clinical presentations of atrophic body gastritis. A positive correlation was obtained between the body atrophy score and the intrinsic factor antibody levels (r = 0.2216, P = 0.0085). Associated autoimmune diseases were present in 25/140 (17.9%) patients, but the prevalence of autoimmune diseases was comparable, irrespective of the clinical presentations.
CONCLUSION: The so-called hallmarks of gastric autoimmunity, particularly in intrinsic factor antibody cannot be usefully employed in defining an autoimmune pattern in the clinical presentations of ABG.
- Citation: Annibale B, Lahner E, Negrini R, Baccini F, Bordi C, Monarca B, Fave GD. Lack of specific association between gastric autoimmunity hallmarks and clinical presentations of atrophic body gastritis. World J Gastroenterol 2005; 11(34): 5351-5357
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5351
Atrophic body gastritis (ABG), particularly in association with pernicious anemia (PA), is believed to be an autoimmune disease, due to the presence of autoantibodies to the secretory elements of the gastric oxyntic mucosa and other associated autoimmune disorders[1,2]. Indeed, parietal cell antibodies (PCA) are detected in 60-90%, and intrinsic factor antibodies (IFA) are found in 50-70% of patients with PA[3-7]. In particular, IFA belonging to the IgG class of immunoglobulins, are considered highly specific to patients with PA, since they have rarely been found in the absence of this condition[8,9].
ABG is usually considered synonymous with the presence of PA, a disorder believed to be most common in individuals of north European descent[10]. However, macrocytic anemia is not the only hematological presentation of ABG. It has been reported, that iron deficiency anemia (IDA) should also be taken into consideration as a presentation symptom of ABG[11]. In fact, in 27% of IDA patients without gastrointestinal symptoms, ABG has been identified as the likely cause of IDA[12]. Furthermore, it is well known, that patients with ABG often complain of dyspepsia, even without hematological disorders[13].
It is now apparent that Helicobacter pylori (H pylori) is involved in the induction of ABG[14]. In fact, it has recently been reported that two-thirds of ABG patients and a large percentage of PA patients present evidence of H pylori infection[15,16]. At the same time, it is well established that H pylori infection can induce gastric autoimmunity, since the presence of the bacteria leads to the production of antibodies cross-reacting with human gastric mucosa[17,18]. Recently, it has been reported that the progression of the severity of gastric corporal atrophy is inversely correlated with circulating IgG to H pylori and positively with the titer of antibodies to parietal cells[15]. Indeed, most of the studies regarding the role of IFA in ABG and PA date back to the early seventies[4,5,8,9], and were thus performed before a histological consensus classification of gastritis (e.g., Sydney System) was developed, and the role of H pylori infection in this condition was recognized. To our knowledge, studies that evaluate the pattern of gastric autoimmune autoantibodies in relation to H pylori status and presentation symptoms of atrophic body gastritis are scarce. Thus, the aim of the present study is to investigate the relationships between the main clinical presentations of ABG and gastric autoimmune phenomena in a consecutive series of patients with ABG.
We included 140 patients with ABG diagnosed in consecutive outpatients referred to our Gastroenterology Department from the Hematology Department for evaluation of their anemia (n = 122, either macrocytic or iron-deficiency anemia) or attending our department for unexplained long-standing dyspepsia (n = 18), in application of previously reported screening programs[11,19]. The ABG patients with dyspepsia did not show any type of anemia. Patients taking anti-secretory drugs or with a history of previous gastric and/or intestinal surgery were carefully excluded. Other causes of anemia (e.g., myelodysplasia, neoplasia, blood loss, epistaxis, heavy menstrual loss, fecal occult blood positivity, cirrhosis) were also carefully excluded, as previously reported[12]. None of the patients were vegetarians and none presented with disorders associated with the small bowel.
All ABG patients had a detailed initial assessment including fasting gastrin, gastroscopy with gastric antrum and body mucosa biopsies as well as basal and peak acid output to pentagastrin (PAO). A serum sample was taken for detection of antibodies against intrinsic factor, parietal cells and H pylori. Anamnestic data were obtained from each patient by a scrupulously detailed clinical interview and, whenever possible by checking medical records, for the presence of associated autoimmune diseases. All patients gave informed consent to the study, which was approved by a local ethics committee.
Diagnosis of atrophic body gastritis The diagnosis of ABG was based on the following criteria: fasting hypergastrinemia, pentagastrin-resistant achlorhydria or hypochlorhydria (PAO lower than 13 mEq/h) and histological confirmation of gastric body mucosa atrophy (see section on histological procedure).
Diagnosis of pernicious anemia PA was defined as a hemoglobin concentration < 14 g/dL for men (normal range 14-18 g/dL) and < 12 g/dL for women (normal range 12-16 g/dL), mean corpuscular volume (MCV) ≥ 100 fL, low level of vitamin B12 (n.v. 190-950 pg/mL), response to vitamin B12 therapy, and histological confirmation of gastric body mucosa atrophy, as described elsewhere[11,16].
Diagnosis of iron deficiency anemia Iron deficiency anemia was defined as hemoglobin concentration < 14 g/dL for men (normal range 14-18 g/dL) and 12 g/dL for women (normal range 12-16 g/dL), MCV < 80 fL (normal range 80-100 fL) and low serum ferritin (normal range 30-180 ng/mL), as previously described[11,12].
Gastric acid secretion studies PAO (6 µg subcutaneous, Peptavlon, ICI, UK) was determined as previously described[20]. The median reference value for PAO in our laboratory was 31.1 mEq/h (range 13-44.4 mEq/h).
Serological testing Fasting gastrin levels were evaluated by a specific radioimmunoassay using antibody no. 4562 (kindly provided by Professor JF Rehfeld Copenhagen, Denmark), as described elsewhere[20]. Normal values were ≤ 40 pg/mL.
H pylori antibodies were detected by competitive ELISA based on a mAb against 64-ku H pylori antigen, as previously described[21]. Antibody concentration was expressed as U/mL. Negative values were < 450 U/mL.
Pepsinogen I levels were measured using a commercial RIA kit (Pepsik, Sorin, Saluggia, Italy) as previously reported[22]. The normal reference values were 20-80 ng/mL.
Parietal cell antibodies (PCA) were determined on serum using a solid phase immunosorbent assay commercial kit (AUTOSTAT, Cogent Diagnostic Ltd., Edinburgh, UK), as described elsewhere[15,19]. Intrinsic factor antibody (IFA) IgG was determined by an indirect ELISA using highly purified porcine IF (Sigma-Aldrich, Saint Louis, USA) as a target. Microwells were overcoated with 10% normal rabbit serum to avoid nonspecific binding of human IgG. Antibody concentration was calculated by a calibration curve and expressed as arbitrary U/mL. Values >2 U/mL were considered positive for IFA[23].
All patients underwent upper gastrointestinal endoscopy with three antral biopsy samples (smaller and greater curve, anterior or posterior) and three from the midbody along the greater curve, taken with a standard biopsy forceps[24]. The specimens were immediately fixed in Bouin’s solution for 4-8 h, at room temperature, rinsed in 0.1 mol/L phosphate-buffered saline, pH 7.4 and routinely processed to wax. Serial paraffin sections (5 µm) were stained with hematoxylin and eosin for conventional histological evaluation and with Giemsa stain for H pylori determination.
Assessment of the degree of gastritis was performed according to the updated Sydney System[25]. The following scores were assigned to each graded variable: 0 for absence and 1, 2, 3, for mild, moderate or severe presence, respectively. Atrophy of gastric mucosa was defined as focal or complete replacement of oxyntic glands by metaplastic pyloric or intestinal glands.
The biopsies were examined independently by two histopathologists, unaware of the clinical data of the patients. In case of any disagreement, the relevant biopsies were re-examined simultaneously by both histopathologists, until an agreement was reached.
H pylori status of ABG patients was evaluated considering both tissue stain and serology, as described elsewhere[15]: patients were defined as H pylori negative/negative (group I) when both histology and serology were negative for H pylori, as H pylori negative/positive (group II) when H pylori was not detected at histology but circulating antibodies to H pylori exceeded the reference value, and as H pylori positive/ positive (group III) when they were found to be positive at histology and circulating antibodies to H pylori exceeded the reference values.
Data were expressed as mean ± SE and/or median (range) and evaluated by appropriate statistical tests (Student t-test or Wilcoxon test). Subgroups (percentages) of the population were compared by Fisher’s exact test. P < 0.05 was considered statistically significant.
ABG patients presenting with PA, IDA, and dyspepsia were not homogeneous as far as gender, age, gastrin and pepsinogen I levels, and body atrophy score were concerned (Table 1). Whereas gender was equally distributed in ABG patients presenting with PA and dyspepsia, ABG patients with IDA were predominantly female. ABG patients with PA were significantly older and had higher levels of gastrin and lower levels of pepsinogen I, compared to those with IDA and dyspepsia. Moreover, ABG patients with PA also had a higher body atrophy score than those with IDA and dyspepsia. However, it is of interest that all ABG patients, regardless of the three clinical presentations, showed comparable stimulated acid secretion values. It is also worthwhile pointing out that the serological levels of vitamin B12 were as expected, significantly lower in ABG patients with PA, albeit also the patients presenting with IDA and dyspepsia had median levels of B12, close to the lower normal value. Finally, H pylori positivity was significantly lower in the PA group than in the IDA and dyspeptic patients.
| Perniciousanemia | Irondeficiencyanemia | Dyspepsia | |
| Demographic and clinical features | |||
| Patients (%) | 65 (46.5) | 57 (40.7) | 18 (12.8) |
| Gender (M/F) | 34/31a | 8/49e | 7/11 |
| Age, yr | 63 (30-83)a | 46 (22-78) | 56 (31-81) |
| Associated autoimmune | 11 (16.9) | 10 (17.5) | 4 (22.2) |
| disease, n (%) | |||
| Biochemical features and gastric autoantibodies | |||
| Gastrin, pg/mL | 500 (55-2 700)b | 375 (50-1 650) | 370 (49-950) |
| Pepsinogen I, ng/mL | 5 (0-68) b | 10 (0-72) | 10 (2-84) |
| PAO1, mEq/h | 0 (0-11.6) | 0 (0-11.9) | 0 (0-12.5) |
| PCA positive2, n (%) | 46 (70.7) | 38 (66.6) | 15 (83) |
| IFA positive3, n (%) | 32 (49.2)a | 12 (21.1) | 5 (27.8) |
| Vitamin B12, pg/mL | 80 (13-394)ad | 200 (80-701) | 195 (100-760) |
| Histological features | |||
| Body atrophy, n (%) | 65 (100) | 57 (100) | 18 (100) |
| Body atrophy score | 2.83 ± 0.06ad | 2.1 ± 0.10 | 1.94 ± 1.7 |
| Antral atrophy, n (%) | 15 (23.1) | 14 (24.6) | 7 (38.9) |
| Antral atrophy score | 1.47 ± 0.13 | 1.29 ± 0.13 | 1.29 ± 0.18 |
| Antrum spared, n (%) | 30 (46.1) | 25 (43.8) | 4 (22.2) |
| H pylori positive4, n (%) | 40 (61.5)ac | 51 (89.5) | 16 (88.9) |
Among the 140 ABG patients, associated autoimmune diseases were observed in only 25 (17.9%, 13 females, 12 males, median age 52 years, range 26-76 years), who presented autoimmune thyroid disease (n = 10), alopecia (n = 6), vitiligo (n = 5), psoriasis (n = 2), rheumatoid arthritis (n = 1) and type I diabetes mellitus (n = 1). However, the prevalence of autoimmune diseases was similar in ABG patients, irrespective of the clinical presentations.
As far as gastric autoimmune antibodies are concerned, the prevalence of PCA was equally distributed among the different clinical presentations, whereas the prevalence of IFA positive cases was significantly higher in ABG patients with PA than in those with IDA and dyspepsia (P = 0.001). Moreover, in order to establish whether gastric autoantibodies were specifically related to any of the clinical presentations, ABG patients were classified on the basis of their positivity for PCA alone, for IFA alone, and positivity and negativity for both antibodies. However, no specific pattern of autoantibodies was related to the clinical presentations of ABG (data not shown).
Nevertheless, the presence of the “spared” antrum, a typical histological aspect of autoimmune gastritis, was similar in ABG patients presenting with PA and IDA (46.1% and 43.8%, respectively), but lower (22.2%) in ABG patients presenting with dyspepsia, albeit statistical significance was not reached. Moreover, the positivity of PCA and IFA was equally distributed in ABG patients with the “spared” antrum and those with antral involvement (data not shown).
Among the 140 ABG patients, 99 (70.7%, 32 males, 67 females, median age 54 years, range 22-83 years) had a positive PCA titer. However, no significant differences were observed between PCA positive and negative ABG patients in any of the following parameters: age, fasting gastrin and pepsinogen I levels, clinical presentation, stimulated acid secretion, and associated autoimmune diseases (data not shown).
In contrast, IFA were positive in 49 of the 140 ABG patients (35%, 18 males, 31 females, median age 58 years, range 26-83). The median level in IFA positive patients was 7.5 U/mL (range 2.1-40 U/mL) vs 0.8 U/mL (range 0-1.95 U/mL) in IFA negative patients (P < 0.0001). Clinical, serological and histological features of IFA positive and negative patients are shown in Table 2. Male/female ratio, age, and the presence of associated autoimmune diseases were similar in IFA positive and negative patients. Among the 25 ABG patients with autoimmune diseases, only 10 (40%) had positive IFA levels: 5 of them with PA, 4 with IDA and 1 with dyspepsia.
| . | IFA positive | IFA negative | P |
| Demographic and clinical features | |||
| Patients (%) | 49 (35) | 91 (65) | |
| Gender (M/F) | 18/31 | 31/60 | NS |
| Age, yr, median (range) | 58 (26-83) | 55 (22-78) | NS |
| Pernicious anemia, n (%) | 32 (65.3) | 33 (36.3) | P = 0.0013 |
| Iron deficiency anemia, n (%) 12 (24.5) | 45 (49.4) | P = 0.0042 | |
| Dyspepsia, n (%) | 5 (10.2) | 13 (14.3) | NS |
| Associated autoimmune | 10 (20.4) | 15 (16.5) | NS |
| disease, n (%) | |||
| Biochemical features and gastric autoantibodies | |||
| PCA positive1, n pts (%) | 39 (80) | 70 (77) | NS |
| Gastrin (pg/ mL) | 500 (55-2.500) | 405 (49-2.700) | P = 0.0579 |
| median (range) | |||
| Pepsinogen I (ng/mlL) | 5 (0-72) | 10 (0-84) | P = 0.0027 |
| median (range) | |||
| Histological features | |||
| Body atrophy score | 2.67 ± 0.09 | 2.28 ± 0.08 | P = 0.0028 |
| (mean ± SE) | |||
| Antral atrophy, n (%) | 10 (20.4) | 26 (28.6) | NS |
| Antral atrophy score | 1.2 ± 0.13 | 1.42 ± 0.1 | NS |
| (mean ± SE) | |||
| Antrum spared, n (%) | 22 (44.9) | 37 (40.7) | NS |
| H pylori positive2, n (%) | 37 (76) | 70 (77) | NS |
Moreover, IFA positive patients had a significantly higher body atrophy score than IFA negative patients (2.67 ± 0.09 vs 2.28 ± 0.08, P = 0.0028). As far as the antrum was concerned, the presence of antral atrophy and the “spared” antrum as well as its histological score and its presence did not differ between the two groups.
As expected, the median value of fasting gastrin was higher and the median value of pepsinogen I was lower in IFA positive than in IFA negative patients (P = 0.05 and P = 0.002, respectively). IFA positive patients were more likely to present with PA (P = 0.0013 vs IFA negative, Fisher’s test), while IFA negative patients presented more frequently with IDA (P = 0.0042 vs IFA positive, Fisher’s test), even though 24.5% of IDA patients and 10.2% of dyspeptic patients had positive IFA levels. It is of interest that the distribution of PCA positive patients was similar between the IFA positive and negative patients.
Considering all the 140 ABG patients, there was a positive, statistically significant correlation between the histological score of body atrophy and the IFA levels (r = 0.2216, P = 0.0085, Spearman rank correlation), suggesting that the progression of oxyntic cell damage could lead to an increase in the production of IFA (Figure 1).
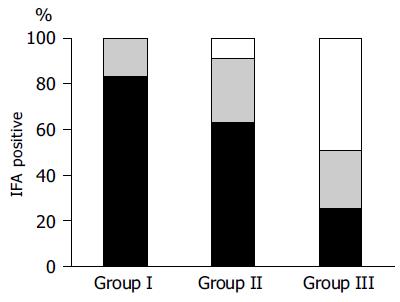
Among the 140 ABG patients, 33 (23.6%) were H pylori negative/negative (group I), 76 (54.3%) were H pylori negative/positive (group II) and 31 (22.1%) were H pylori positive/positive (group III).
IFA were found to be positive in 12 out of 33 (36.4%) group I patients, in 33 out of 76 (43.5%) group II patients, and in 4 out of 31 (12.9%) group III patients (Figure 2). The proportion of IFA positive patients were significantly higher in groups I and II than in group III (Fisher’s test: P = 0.043 and P = 0.003, respectively). Also the mean level of IFA was significantly higher in groups I and II than in group III (P = 0.01 and P = 0.004, respectively).
The relationships between IFA positivity, H pylori status and clinical presentations observed in ABG patients are shown in Figure 3. The majority of IFA positive ABG patients had PA, and most of the PA patients were in the H pylori negative group. Albeit, at the same time, the positivity for IFA was not exclusive to PA patients, occurring in a considerable percentage of ABG patients presenting with IDA or even with dyspepsia. ABG patients with these two latter clinical presentations were mainly H pylori positive.
Two mechanisms are generally considered to induce body mucosa atrophy. On the one hand, the autoimmune pathogenesis of atrophic gastritis is well accepted, and PA is considered as its most characteristic clinical presentation[13,14]. On the other hand, it has been recognized that long-standing H pylori infection may induce atrophy of the oxyntic mucosa, which may clinically present with long-standing dyspepsia or IDA[11,15,26].
The main finding from this study is that gastric autoantibodies are present in ABG patients irrespective of the clinical presentations. In fact, the presence of gastric autoantibodies is not exclusive of pernicious anemia, but is also found in ABG patients with iron-deficiency anemia and dyspepsia. In particular, PCA are certainly present in the vast majority of ABG patients irrespective of the clinical presentations. In contrast, the presence of IFA is significantly higher in PA patients (50%), but is also detectable in >20% of IDA and dyspeptic ABG patients. In our opinion, the results obtained are due mainly to the original methodological approach to the diagnosis of ABG, which is based on a screening program for the early detection of this condition in patients with anemia and/or long-standing dyspepsia[11,12]. Thus, the study population comprises a wider clinical spectrum of this condition than in previous studies[8,9], thus avoiding a potential bias due to the a priori-selection of the patients. For example, in our study the prevalence of IFA, in all ABG patients, was relatively low (35%), but considering only those with PA, the positivity of IFA reached 50%, which is similar to that described in previous reports[3-7]. In the present study, the classification of ABG patients based on the absence and/or the co-existing positivity and negativity of IFA and PCA failed to identify any specific pattern of gastric autoantibodies related to the clinical presentations of ABG.
Another important finding from the present study was the positive correlation between IFA levels and the histological score of gastric body atrophy in ABG patients, suggesting that progression of the oxyntic mucosa damage leads to an increase in the production of IFA, as previously observed for PCA[15]. Thus, our findings suggest that the positivity of IFA and/or PCA may be an expression of the degree of oxyntic mucosa damage in each patient, rather than a specific hallmark of autoimmunity.
It is of interest, that one-third of the ABG patients with positivity for IFA do not have PA, but present with IDA and dyspepsia. In previous studies, IFA are considered highly specific for PA[8,9] and only appear once when PA develops, thus assigning them a pathogenetic role in this condition[27]. Furthermore, it has been recognized that in PA patients, the co-existence of iron deficiency can occur in up to 20% of patients at initial diagnosis and in another 20% of patients during a 2-year follow-up,[28], indicating that both cobalamin and iron deficiency may overlap during the failure of oxyntic function. On the other hand, in our ABG patients presenting with iron deficiency anemia or dyspepsia, the serum levels of vitamin B12 were relatively low (Table 1), suggesting the presence of “latent” pernicious anemia in a proportion of ABG patients. Thus, chronic oxyntic failure may be present with a variable spectrum of hematological disorders depending on the age at onset of the disease, and gender, as observed elsewhere[16,29].
The positivity of IFA, like the positivity of PCA in patients with ABG and PA, together with the association of autoimmune disorders, is considered to be a hallmark of the autoimmune origin of these conditions[1,2]. In our study, < 20% of patients with ABG had associated autoimmune disease and <50% of patients with ABG and autoimmune disease were IFA positive, only half of them having associated PA. However, the presence of associated autoimmune disease was similarly distributed in ABG patients with PA, IDA, and dyspepsia. Thus, these findings further highlight that these characteristics, previously considered specific, are not reliable in defining a specific subgroup of patients with autoimmune ABG.
Furthermore, it is well known that autoimmune gastritis has no distinctive histological features[30]. The “spared” antrum is often considered to be associated with autoimmune gastritis, which in fact is generally body-restricted[31]. However, this histological finding is not peculiar to any of the clinical presentations of ABG (Table 1).
Taking H pylori infection into consideration, results of this study suggest a possible relationship between the positivity of IFA and the time course of H pylori infection (Figure 2). Indeed, at an earlier stage of H pylori infection, when the organism is still detectable by tissue staining, the proportion of IFA positive ABG patients and their median level of IFA are significantly lower than that of those patients presenting with a long-standing infection, in whom only immunological memory or even no sign of infection is detectable. These data show that there is a positive correlation between the progression of body atrophy and production of IFA, since it has been reported that evidence of H pylori infection disappears parallel to the progression of body mucosa damage[15,32].
In fact, in ABG patients, oxyntic mucosa damage may be triggered by H pylori infection[14,33], and may be, as a consequence, exposed to autoantigens on these damaged cells. In animal models, it has been shown that gastric autoimmunity could be the consequence of a local inflammatory response induced by the local expression of pro-inflammatory molecules[34,35]. This hypothesis is supported by the findings of Ma et al[36] who have observed a positive correlation between PCA and H pylori antibodies in patients with PA. Furthermore, Claeys et al[37] also showed a positive correlation between antibodies against H+, K+-ATPase and antibodies against H pylori in patients with PA, suggesting that mixed forms of bacterial and autoimmune gastritis might occur. Recently, it has also been reported that, in genetically susceptible individuals, H pylori infection may trigger the development of gastric autoimmunity via molecular mimicry[38]. Thus, these data suggest that the sole autoimmune origin of ABG may be questionable, and that the role of H pylori infection, as an initial trigger of chronic damage to the oxyntic mucosa, should be taken into consideration, regardless of the clinical presentations of ABG.
In conclusion, the so-called hallmarks of gastric autoimmunity, particularly in IFA, cannot be usefully employed in defining an autoimmune pattern in the clinical presentations of ABG.
The authors thank Mrs. Amelia Pasquali for skilled technical support and Mrs. Marian Shields for revision of English style and editorial assistance.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Toh BH, Gleeson PA, Whittingham S, Van Driel IR. Autoim-mune gastritis and pernicious anemia. In: Rose NR, Mackay IR, Eds. The Autoimmune Diseases, 3rd edition. San Diego, London, Boston, New York, Sydney, Tokyo, Toronto, Academic Press 1998; 459-476. |
| 2. | Babio BM. Erythrocyte disorders: Anemias related to distur-bance of DNA synthesis (megaloblastic anaemias). In: Williams WJ, Beutler E, Erslev AJ, Lichtman MA, eds. Haematology, 4th edition. New York 1998; 453-481. |
| 3. | Wintrobe MM, Lee GR, Boggs DR, Bithell TC, Foerster J, Athens JW, Lukens JN. Megaloblastic and nonmegaloblastic mac-rocytic anaemias. In: Wintrobe MM, Lee GR, Boggs DR, Bithell TC, Foerster J, Athens JW, Lukens JN, eds. Clinical Haematology, 8th edition, Philadelphia 1981; 559-604. |
| 4. | Irvine WJ, Cullen DR, Scarth L, Simpson JD. Intrinsic-factor secretion assessed by direct radioimmunoassay and by total-body counting in patients with achlorhydria and in acid secretors. Lancet. 1968;2:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Fisher JM, Taylor KB. The significance of gastric antibodies. Br J Haematol. 1971;20:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Kapadia CR, Donaldson RM. Disorders of cobalamin (vitamin B12) absorption and transport. Annu Rev Med. 1985;36:93-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Carmel R. Reassessment of the relative prevalences of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: influence of patient age and race. Clin Exp Immunol. 1992;89:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Hift W, Moshal MG, Pillay K. Megaloblastic anaemia, achlorhydria, low intrinsic factor, and intrinsic-factor antibodies in the absence of pernicious anaemia. Lancet. 1973;1:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Rose MS, Doniach D, Chanarin I, Brostoff J, Ardeman S. Intrinsic-factor antibodies in absence of pernicious anaemia. 3-7 year follow-up. Lancet. 1970;2:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Scheuner M, Yang H, Rotter JI. Gastrointestinal manifesta-tions of specific genetic disorders. Textbook of Gastroenterology, 2nd edition, Philadelphia: JB Lippincott Company 1995; 2380-2419. |
| 11. | Marignani M, Delle Fave G, Mecarocci S, Bordi C, Angeletti S, D'Ambra G, Aprile MR, Corleto VD, Monarca B, Annibale B. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: a prospective screening study. Am J Gastroenterol. 1999;94:766-772. [PubMed] |
| 12. | Annibale B, Capurso G, Chistolini A, D'Ambra G, DiGiulio E, Monarca B, DelleFave G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001;111:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Yardley JH, Hendrix TR; Gastritis, gastropathy, duodenitis, and associated ulcerative lesions. In: Yamada T Ed. . |
| 14. | Textobook of Gastroenterology, 1st Edition Lippincott, Will-iams & Wilkins, Philadelphia. New York: Baltimore 1999; 1463-1499. |
| 15. | Peterson WL, Graham DY; Helicobacter pylori. In: Feldman M, Friedman LS, Sleisenger MH Eds. Sleisenger & Fordtran's. . [DOI] [Full Text] |
| 16. | Gastrointestinal and Liver Disease: pathophysiology, diagnosis, management, 7th edition, Philadelphia, London, New York, St. Louis, Sydney, Toronto. 2002;732-746. |
| 17. | Annibale B, Negrini R, Caruana P, Lahner E, Grossi C, Bordi C, Delle Fave G. Two-thirds of atrophic body gastritis patients have evidence of Helicobacter pylori infection. Helicobacter. 2001;6:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Annibale B, Lahner E, Bordi C, Martino G, Caruana P, Grossi C, Negrini R, Delle Fave G. Role of Helicobacter pylori infection in pernicious anaemia. Dig Liver Dis. 2000;32:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Appelmelk BJ, Faller G, Claeys D, Kirchner T, Vandenbroucke-Grauls CM. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol Today. 1998;19:296-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Negrini R, Lisato L, Zanella I, Cavazzini L, Gullini S, Villanacci V, Poiesi C, Albertini A, Ghielmi S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101:437-445. [PubMed] |
| 21. | Annibale B, Marignani M, Azzoni C, D'Ambra G, Caruana P, D'Adda T, Delle Fave G, Bordi C. Atrophic body gastritis: distinct features associated with Helicobacter pylori infection. Helicobacter. 1997;2:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Delle Fave G, Kohn A, De Magistris L, Annibale B, Bruzzone R, Sparvoli C, Severi C, Torsoli A. Effects of bombesin on gastrin and gastric acid secretion in patients with duodenal ulcer. Gut. 1983;24:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Negrini R, Zanella I, Savio A, Poiesi C, Verardi R, Ghielmi S, Alberini A, Sangaletti O, Lazzaroni M, Bianchi Porro G. Se-rodiagnosis of Helicobacter pylori-associated gastritis with a monoclonal antibody competitive enzyme-linked immunosorbent assay. Scand J Gastroenterol. 1992;27:599-605. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Annibale B, De Magistris L, Corleto V, D'Ambra G, Marignani M, Iannoni C, Delle Fave G. Zollinger-Ellison syndrome and antral G-cell hyperfunction in patients with resistant duodenal ulcer disease. Aliment Pharmacol Ther. 1994;8:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Negrini R, Benedini S, Annibale B, Delle Fave G, Vaira D, Bertolat G, Savio A. Antibodies against intrinsic factor are induced by Helicobacter pylori infection. Gut. 2000;47:A87-88. |
| 26. | Bordi C, Annibale B, Azzoni C, Marignani M, Ferraro G, Antonelli G, D'Adda T, D'Ambra G, Delle Fave G. Endocrine cell growths in atrophic body gastritis. Critical evaluation of a histological classification. J Pathol. 1997;182:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3550] [Article Influence: 122.4] [Reference Citation Analysis (3)] |
| 28. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 496] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 29. | Roitt IM, Doniach D, Shapland C. Autoimmunity in pernicious anemia and atrophic gastritis. Ann N Y Acad Sci. 1965;124:644-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Carmel R, Weiner JM, Johnson CS. Iron deficiency occurs frequently in patients with pernicious anemia. JAMA. 1987;257:1081-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Capurso G, Marignani M, Delle Fave G, Annibale B. Iron-deficiency anemia in premenopausal women: why not consider atrophic body gastritis and Helicobacter pylori role? Am J Gastroenterol. 1999;94:3084-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Capella C, Fiocca R, Cornaggia M, Rindi G, Moratti R, Solcia E. Autoimmune Gastritis. In: Gastritis, Graham DY, Genta RM, Dixon MF (Eds), Philadelphia, Lippincott Williams & Wilkins 1999; 79-96. |
| 33. | Lee EL, Feldman M. Gastritis and other gastropathies. In: Sleisenger & Fordtran's Gastrointestinal and Liver Disease: pathophysiology, diagnosis, management. Feldman M, Friedman LS, Sleisenger MH (eds). 7th edition. Philadelphia; Saunders 2002; 810-827. |
| 34. | Karnes WE, Samloff IM, Siurala M, Kekki M, Sipponen P, Kim SW, Walsh JH. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167-174. [PubMed] |
| 35. | Lahner E, Bordi C, Di Giulio E, Caruana P, D'Ambra G, Milione M, Grossi C, Delle Fave G, Annibale B. Role of Helicobacter pylori serology in atrophic body gastritis after eradication treatment. Aliment Pharmacol Ther. 2002;16:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Alderuccio F, Sentry JW, Marshall AC, Biondo M, Toh BH. Animal models of human disease: experimental autoimmune gastritis--a model for autoimmune gastritis and pernicious anemia. Clin Immunol. 2002;102:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Cróinín TO, Clyne M, Appelmelk BJ, Drumm B. Antigastric autoantibodies in ferrets naturally infected with Helicobacter mustelae. Infect Immun. 2001;69:2708-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Ma JY, Borch K, Sj�strand SE, Janzon L, Mardh S. Positive correlation between H,K-Adenosine Triphosphatase autoan-tibodies and Helicobacter pylori antibodies in patients with pernicious anaemia. Scand J Gastroenterol. 1994;29:961-965. [RCA] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D'Elios MM. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |