Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5283
Revised: March 20, 2005
Accepted: March 23, 2005
Published online: September 14, 2005
AIM: To investigate the long-term consequences of chemotherapy-related HBV reactivation in patients with lymphoma.
METHODS: This study was based on the database of published prospective study evaluating HBV reactivation in HBV lymphoma patients during chemotherapy. Deteriorated liver reserve (DLR) was defined as development of either one of the following conditions during follow-up: (1) newly onset parenchyma liver disease, splenomegaly or ascites without evidence of lymphoma involvement; (2) decrease of the ratio (albumin/globulin ratio) to less than 0.8 or increase of the ratio of INR of prothrombin time to larger than 1.2 without evidence of malnutrition or infection. Liver cirrhosis was diagnosed by imaging studies.
RESULTS: A total of 49 patients were included. The median follow-up was 6.2 years (range, 3.9-8.1 years). There were 31 patients with and 18 patients without HBV reactivation. Although there was no difference of overall survival (OS) and chemotherapy response rate between the two groups, DLR developed more frequently in patients with HBV reactivation (48.4% vs 16.7%; P = 0.0342). Among the HBV reactivators, HBV genotype C was associated with a higher risk of developing DLR (P = 0.0768) and liver cirrhosis (P = 0.003). Four of five patients with sustained high titer of HBV DNA and two of three patients with multiple HBV reactivation developed DLR. Further, patients with a sustained high titer of HBV DNA had the shortest OS among the HBV reactivators (P = 0.0000). No patients in the non-HBV reactivation group developed hepatic failure or liver cirrhosis.
CONCLUSION: Chemotherapy-related HBV reactivation is associated with the long-term effect of deterioration of hepatic function.
- Citation: Su WP, Wen CC, Hsiung CA, Su IJ, Cheng AL, Chang MC, Tsao CJ, Kao WY, Uen WC, Hsu C, Hsu CH, Lu YS, Tien HF, Chao TY, Chen LT, Whang-Peng J, Chen PJ. Long-term hepatic consequences of chemotherapy-related HBV reactivation in lymphoma patients. World J Gastroenterol 2005; 11(34): 5283-5288
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5283
Reactivation of HBV is a well-recognized complication in patients with chronic HBV infection, who receive cytotoxic chemotherapy or immunotherapy management for cancers[1-16] This complication is especially important in Taiwan, Hong Kong, Singapore and Mainland China, which have high HBV carrier rates of approximately 15-20% in the general population. Although the reported frequency of HBV reactivation in hepatitis B surface antigen (HBsAg)-positive non-Hodgkin’s lymphoma (NHL) patients undergoing cancer chemotherapy has ranged widely from 14% to 72%[8-10], our recent prospective study indicated a HBV reactivation rate of around 70% in patients receiving conventional glucocorticoids-containing systemic chemotherapy[17]. In the near-term, hepatitis flare-up in patients undergoing chemotherapy may jeopardize scheduled chemotherapy or cause severe hepatic damage and even lethal hepatic failure of the patients. However, for survivors of lymphoma who have HBV reactivation during chemotherapy, the long-term hepatic consequences have not been elucidated.
In this study, we analyzed the long-term hepatic consequence in HBV lymphoma patients with HBV reactivation during chemotherapy by using the database of the TCOG (Taiwan Cooperative Oncology Group)-1495, initiated in 1995 and a prospective multi-center randomized clinical study, to evaluate HBV reactivation in HBsAg (+)-lymphoma patients[17]. Most of the patients in this study were followed up for more than 6 years. Our results indicated that though there is no difference in overall survival (OS) in the patients with or without HBV reactivation, HBV reactivation during systemic chemotherapy may have long-term adverse effect on the livers of the lymphoma survivors.
This retrospective study used data collected during the long-term follow-up of patients enrolled in a multi-center randomized clinical study conducted from November 1995 to February 2000, which compared steroid-containing and steroid-free chemotherapy in HBsAg (+)-lymphoma patients (T-1495)[17]. All 49 patients who participated in that prospective trial were included in this study. HBV DNA, hepatitis B e antigen (HBeAg) status, HBV genotype and biochemical data (albumin, globulin, bilirubin in total and direct forms, serum aspartate aminotransaminase, serum alanine aminotransaminase (ALT), and prothrombin time (PT)) were regularly examined before treatment and every 2 wk during chemotherapy. All these exams except HBV studies were regularly checked every 2 mo during follow-up. Imaging studies (i.e., abdominal ultrasonography and computed tomography) were performed every 3 mo during chemotherapy and annually during the long-term follow-up.
Briefly, HBV DNA was tested in duplicate using the Chiron bDNA assay (VERSANT HBV DNA assay, Chiron Diagnostics, Emeryville, CA, USA). This assay has a sensitivity of 2.5 pg/mL. HBV genotypes were determined using PCR-restriction fragment length polymorphism (RFLP) of the surface gene of HBV[18]. Six genotypes (A-F) of HBV could be identified by the restriction patterns of DNA fragments.
Clinical hepatitis flare-up was defined as a threefold or greater increase in serum ALT level that exceeded 100 IU/L. The hepatitis or hepatitis flare-up was attributed to reactivation of chronic hepatitis B, when there was a five-fold or higher elevation of serum HBV DNA compared with the pre-chemotherapy baseline level or re-appearance of HBV DNA or HBeAg in the serum. Liver biopsy was electively performed to determine the etiology of hepatitis in this study. Only six patients received liver biopsy before or after chemotherapy. Three patients who developed HBV reactivation after early 1999 were treated with lamivudine.
The HBV reactivation pattern was categorized into three groups as transient, protracted and multiple reactivations[17]. Transient pattern was characterized by a rapid and transient surge of HBV DNA, which usually resolved in a few weeks. Protracted HBV reactivation was defined as a prolonged period of persistently high HBV DNA titer. Some patients with protracted HBV reactivation finally achieved resolution after several bouts of clinical hepatitis (protracted/resolved), while others maintained a high-titer of HBV DNA status throughout the entire period of chemotherapy (protracted/ unresolved). Multiple HBV reactivations were characterized by repeated HBV increment after apparent resolution of previous episodes.
In this retrospective study, hepatic images, albumin/globulin ratio (A/G ratio)[19-22] and international normalized ratio (INR) of PT[23-25] were used for the evaluation of liver status. Deteriorated liver reserve (DLR) was defined as the development of either one of the following conditions during the following period: (1) New onset of parenchyma liver disease, liver cirrhosis, splenomegaly or ascites[26] without evidence of lymphoma involvement of the liver; (2) Decrease of the ratio (A/G ratio) to less than 0.8 [(post-chemotherapy A/G ratio)/(pre-chemotherapy A/G ratio)] or increase of the ratio of INR of PT [(post-chemotherapy INR)/(pre-chemotherapy INR)] to larger than 1.2 without evidence of malnutrition or infection status.
All analyses were carried out according to the intent-to-treat principle. Survival curves were estimated by the Kaplan-Meier method. Chi-square test was used to compare variables between patients with and without HBV reactivation. A two-tailed P value less than 0.05 was considered statistically significant. SPSS statistical software (version 10.0) was used for the analysis in this study.
The baseline characteristics of the NHL patients according to the occurrence HBV reactivation are shown in Table 1. The median follow-up of the patients was 6.2 years (range, 3.9-8.1 years). There were 31 patients (63.3%) in the HBV reactivation group and 18 patients (36.7%) in the non-HBV reactivation group. Among 31 patients with clinical HBV reactivation, only one patient had his liver biopsied to confirm the diagnosis. Patients who were more than 45 years old or those who had a lower pre-chemotherapy HBV DNA load (< 100 copy numbers) had a significant tendency to develop HBV reactivation (P = 0.019 and P = 0.010, respectively). The likelihood of developing HBV reactivation was slightly higher in HBeAg (-) patients (P = 0.0841). Gender and HBV genotype did not contribute to HBV reactivation.
| HBV reactivation (-) | HBV reactivation (+) | |||||
| Total | n | % | n | % | P | |
| Number of patients | 49 | 18 | 36.7 | 31 | 63.3 | |
| Median follow-up (yr) | 6.2 | 5.4 | 6.7 | |||
| Age (yr) | ||||||
| ≤45 | 24 | 13 | 54.2 | 11 | 45.8 | 0.019 |
| >45 | 25 | 5 | 20 | 20 | 80 | |
| Sex | ||||||
| Female | 21 | 7 | 33.3 | 14 | 66.7 | 0.7689 |
| Male | 28 | 11 | 39.3 | 17 | 60.1 | |
| HBeAg | ||||||
| Positive | 7 | 5 | 71.4 | 2 | 28.6 | 0.0841 |
| Negative | 42 | 13 | 31 | 29 | 69 | |
| Pre-chemotherapy HBV DNA | ||||||
| ≥100 copy numbers | 11 | 8 | 72.7 | 3 | 27.3 | 0.010 |
| <100 copy numbers | 38 | 10 | 26.3 | 28 | 73.7 | |
| HBV genotype C | ||||||
| Yes | 12 | 5 | 41.7 | 7 | 58.3 | 0.7318 |
| No | 32 | 11 | 34.4 | 21 | 65.6 | |
| Unknown or mixed type | 5 | 2 | 40 | 3 | 60 | |
| Tumor overall response (complete remission and partial remission) | ||||||
| Yes | 26 | 8 | 30.8 | 18 | 69.2 | 0.3897 |
| No | 23 | 10 | 43.5 | 13 | 56.5 | |
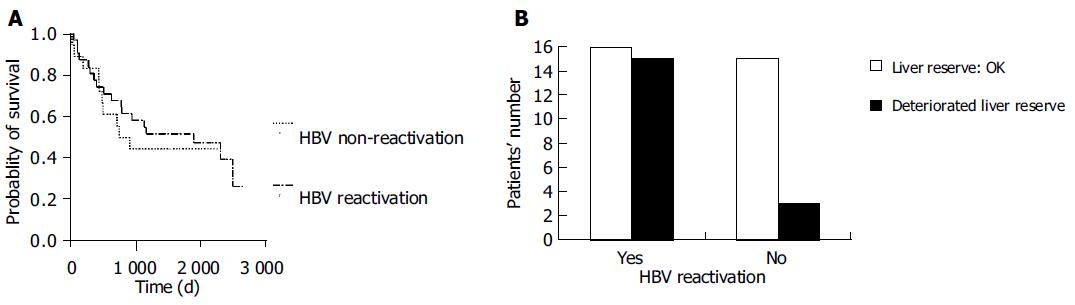
None of the patients developed varices bleeding or hepatocellular carcinoma during the follow-up period. The median survival was 1 886 d in the HBV reactivation group (1 537 ± 188 d, 95%CI as 1 168-1 907 d) and 828 d in the non-HBV reactivation group (1 255 ± 217 d, 95%CI as 829-1 680 d) group. Although there was no difference in treatment response to chemotherapy (P = 0.3897) and OS between patients with and without HBV reactivation (P = 0.601, Figure 1A), DLR was developed more frequently in patients with HBV reactivation (48.4% vs 16.7%, P = 0.0342, Figure 1B).
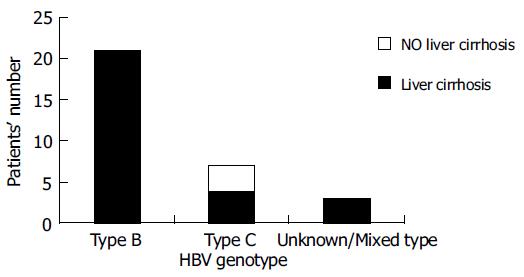
Among the HBV reactivators, those with HBV genotype C had a higher risk of developing DLR (P = 0.0768). No other factor including HBeAg status, age, gender, reactivation pattern and peak value of bilirubin, ALT and HBV DNA level was found to be associated with poor liver outcome (Table 2). Three of thirty-one patients who had viral genotype C had new onset of liver cirrhosis by imaging criteria with Child-Pugh classification A. Genotype C was associated with the development of liver cirrhosis after chemotherapy among lymphoma patients who had HBV reactivation during chemotherapy (P = 0.003, Figure 2).
| DLR (-) | DLR (+) | |||||
| Total | n | % | n | % | P | |
| Number of patients | 31 | 16 | 51.6 | 15 | 48.4 | |
| Age (yr) | ||||||
| ≤45 | 11 | 4 | 36.4 | 7 | 65.6 | 0.2734 |
| >45 | 20 | 12 | 60 | 8 | 40 | |
| Sex | ||||||
| Female | 14 | 7 | 50 | 7 | 50 | 1 |
| Male | 17 | 9 | 52.9 | 8 | 47.1 | |
| GPT>10X | ||||||
| Yes | 19 | 8 | 42.1 | 11 | 57.9 | 0.2734 |
| No | 12 | 8 | 66.7 | 4 | 33.4 | |
| HBeAg | ||||||
| Present | 29 | 16 | 55.2 | 13 | 44.8 | 0.2258 |
| Absent | 2 | 0 | 0 | 2 | 100 | |
| HBV genotype C | ||||||
| No | 21 | 13 | 61.9 | 8 | 38.1 | 0.0768 |
| Yes | 7 | 1 | 14.3 | 6 | 85.7 | |
| Unknown or mixed type | 3 | 2 | 66.7 | 1 | 33.3 | |
| Total bilirubin >5 | ||||||
| Yes | 5 | 1 | 20 | 4 | 80 | 0.1719 |
| No | 26 | 15 | 57.7 | 11 | 42.3 | |
| Peak HBV DNA >100X | ||||||
| Yes | 18 | 9 | 50 | 9 | 50 | 1 |
| No | 13 | 7 | 53.8 | 6 | 46.2 | |
| Patterns of reactivation | ||||||
| Transient | 16 | 10 | 62.5 | 6 | 37.5 | 0.508 |
| Protracted/resolved | 5 | 3 | 60 | 2 | 40 | |
| Protracted/unresolved | 5 | 1 | 20 | 4 | 80 | |
| Multiple reactivation | 3 | 1 | 33.3 | 2 | 66.7 | |
| Unknown | 2 | 1 | 50 | 1 | 50 | |
The characteristics and outcome of patients who developed DLR with patterns of protracted/unresolved (A) and multiple reactivations (B) are listed in Table 3. Four of five patients with pattern A and two of three patients with pattern B developed DLR. All 3 of the 31 patients who died of hepatic failure had protracted/unresolved (A) or multiple reactivation (B) patterns. No cases of hepatic failure or new onset of liver cirrhosis developed in the non-HBV reactivation group.
| Reactivation pattern | Outcome | Cause of death | Type of DLR | Ratio of A/G ratio | Ratio of INR of PT |
| A | Died | Hepatic failure | H | 0.62 | 3.88 |
| A | Died | Hepatic failure | H | 1 | 1.27 |
| A | Died | Cancer progression | N | 0.86 | NA |
| A | Died | Cancer progression | N | 0.6 | 1.38 |
| B | Alive | NA | N | NA | NA |
| B | Died | Hepatic failure/cancer progression | H | NA | 1.36 |
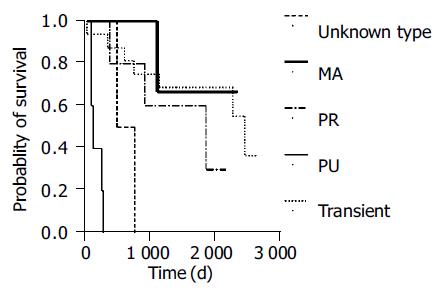
Response to chemotherapy (partial response or complete remission) occurred in 10 of 16 patients with transient reactivation, 4 of 5 patients with protracted/resolved reactivation, 2 of 5 patients with protracted/unresolved flare-up, 2 of 3 patients with multiple reactivation and none of 2 patients with unsure pattern. No correlation was found between reactivation pattern and treatment response (P = 0.325). However, patients with a protracted/unresolved reactivation pattern had shorter OS (189 ± 41 d, 95%CI as 108-269 d, Figure 3, P = 0.000).
In this study, we demonstrated that though there is no survival difference between NHL patients with and without HBV reactivation during chemotherapy, the patients with HBV reactivation do have a long term effect on liver function or liver cirrhosis of the lymphoma survivors by biochemical tests or by imaging modality. HBV genotype C patients may have a higher risk of developing DLR and liver cirrhosis after chemotherapy. Moreover, patients with protracted/unresolved or multiple reactivations are associated with a higher risk of developing DLR and a high incidence of lethal hepatic failure. Furthermore, patients with sustained high titer of HBV DNA leads to poor OS duration compared to other HBV reactivators.
This study had several limitations. DLR after chemotherapy was defined by common biochemical tests with A/G and PT[19-25] and imaging modality because the condition of the patients (i.e. neutropenia and thrombocytopenia during chemotherapy) made routine performance of liver biopsy during lymphoma treatment problematic. The relatively short follow-up duration also limited this study. The longest follow-up duration was only 8.1 years and this might not have been long enough to observe the full clinical course of HBV infection, such as decompensated liver cirrhosis and hepatocellular carcinoma[27]. The small size was another limitation of this study; and in this study, only 31 patients were HBV reactivation patients. So genotype C of HBV only had a borderline of contribution to develop DLR (P = 0.0768). Therefore, further research is needed to establish the relationships among HBV, chemotherapy and host, and to determine the long-term effects of chemotherapy in lymphoma patients who are hepatitis B carriers.
Icteric hepatitis in HBV reactivation during chemotherapy may jeopardize the treatment schedule and thereby increase the risk of lymphoma progression and change the OS of patients with HBV reactivation. No difference was found in the treatment response rate and OS (Figure 1A) of between patients with and without HBV reactivation; however, as shown in Table 3 and Figure 3, patients with a protracted/unresolved reactivation pattern had a shorter OS. Protracted/unresolved reactivation was associated with a higher probability of subsequent hepatic failure or postponed chemotherapy schedule, both of which might contribute to shorter OS. However, in this study, only small proportion patients (4 of 31, 13%) was identified with protracted-unresolved pattern of HBV reactivation; so maybe we should enroll more patients to identify whether the reactivation patterns have influence on the survival.
The worldwide distribution of HBV genotype can be summarized as follows: genotype A is predominant in northern Europe; genotypes B and C are confined to populations with origins in eastern Asia and the Far East; genotype D is found worldwide, but prevails in the Mediterranean area, the Near and Middle East and south Asia; genotype E is indigenous to western sub-Saharan areas; genotype F is likely to be present in populations with origins in the American continent and the newly identified genotype G is found in France and the USA[28]. In Taiwan, the major serotype of HBV is genotype B (about 81%)[29]. Generally speaking, genotype C is associated with more severe liver disease and hepatocellular carcinoma, which may be due to increased risk of HBeAg positivity, higher serum HBV DNA level, less frequent precore stop codon mutation, and a longer immune clearance phase[28].
In this study, patients with HBV genotype C contributed had a higher rate of impaired liver outcome including post-chemotherapy-related liver cirrhosis. However, among HBV re-activators, the proportion with genotype C who became HBeAg seropositive (1 of 7, 14.3%) was not significantly higher compared to those with genotype B (1 of 21, 4.8%) (P = 0.4444, Fisher’s exact test). Besides, the pre-chemotherapy baseline HBV DNA copy numbers of all genotype C patients were not elevated, and all of them were within 100 copies. This suggests that the mechanism of DLR resulting from genotype C in HBV patients might not be fully explained by HBV DNA amount and HBeAg seropositivity. Host immune reaction response to hepatitis virus[19,30] and viral genomic factors[31,32] may be more predominant factors. Further studies are needed to test this hypothesis.
Antiviral drugs (i.e. lamivudine) are now commonly used for treatment and prevention of HBV reactivation in HBsAg seropositive patients undergoing cytotoxic chemotherapy[33,34]. They have shown a complete preventative effect of lamivudine, when used before chemotherapy in HBV carriers with lymphoma. However, there is a lack of data on the response to these antiviral drugs in treating or preventing chemotherapy-related HBV reactivation in cancer patients with different HBV genotypes. Further monitoring of the long-term consequences of HBV infection patients either treated or receiving prophylaxis with antiviral agents during chemotherapy and follow-up of the efficacy of these drugs in different HBV genotypes is needed.
We are very grateful to Lymphoma Committee of Taiwan Cooperative Oncology Group (TCOG) for their support.
Science Editor Guo SY Language Editor Elsevier HK
Co-correspondent: Pei-Jer Chen
| 1. | Galbraith RM, Eddleston AL, Williams R, Zuckerman AJ. Fulminant hepatic failure in leukaemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet. 1975;2:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68:105-112. [PubMed] |
| 3. | Hoofnagle JH, Dusheiko GM, Schafer DF, Jones EA, Micetich KC, Young RC, Costa J. Reactivation of chronic hepatitis B virus infection by cancer chemotherapy. Ann Intern Med. 1982;96:447-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 286] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Thung SN, Gerber MA, Klion F, Gilbert H. Massive hepatic necrosis after chemotherapy withdrawal in a hepatitis B virus carrier. Arch Intern Med. 1985;145:1313-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Lau JY, Lai CL, Lin HJ, Lok AS, Liang RH, Wu PC, Chan TK, Todd D. Fatal reactivation of chronic hepatitis B virus infection following withdrawal of chemotherapy in lymphoma patients. Q J Med. 1989;73:911-917. [PubMed] |
| 6. | Pariente EA, Goudeau A, Dubois F, Degott C, Gluckman E, Devergie A, Brechot C, Schenmetzler C, Bernuau J. Fulminant hepatitis due to reactivation of chronic hepatitis B virus infection after allogeneic bone marrow transplantation. Dig Dis Sci. 1988;33:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Liang RH, Lok AS, Lai CL, Chan TK, Todd D, Chiu EK. Hepatitis B infection in patients with lymphomas. Hematol Oncol. 1990;8:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182-188. [PubMed] |
| 9. | Liaw YF. Hepatitis viruses under immunosuppressive agents. J Gastroenterol Hepatol. 1998;13:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Alexopoulos CG, Vaslamatzis M, Hatzidimitriou G. Prevalence of hepatitis B virus marker positivity and evolution of hepatitis B virus profile, during chemotherapy, in patients with solid tumours. Br J Cancer. 1999;81:69-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology. 1999;46:2925-2930. [PubMed] |
| 12. | Liang R, Lau GK, Kwong YL. Chemotherapy and bone marrow transplantation for cancer patients who are also chronic hepatitis B carriers: a review of the problem. J Clin Oncol. 1999;17:394-398. [PubMed] |
| 13. | Cheng AL. Steroid-free chemotherapy decreases the risk of hepatitis flare-up in hepatitis B virus carriers with non-Hodgkin's lymphoma. Blood. 1996;87:1202. [PubMed] |
| 14. | Kumagai K, Takagi T, Nakamura S, Sawada U, Kura Y, Kodama F, Shimano S, Kudoh I, Nakamura H, Sawada K. Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol. 1997;8 Suppl 1:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, Tosi A, Cassi E. Acute hepatic toxicity during cyclic chemotherapy in non Hodgkin's lymphoma. Haematologica. 1997;82:38-42. [PubMed] |
| 16. | Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, Kao WY, Uen WC, Hsu CH, Tien HF. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 699] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 19. | Triger DR, Wright R. Hyperglobulinaemia in liver disease. Lancet. 1973;1:1494-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 104] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Czaja AJ, Wolf AM, Baggenstoss AH. Clinical assessment of cirrhosis in severe chronic active liver disease: specificity and sensitivity of physical and laboratory findings. Mayo Clin Proc. 1980;55:360-364. [PubMed] |
| 21. | Lin DY, Liaw YF, Chu CM, Chang-Chien CS, Wu CS, Chen PC, Sheen IS. Hepatocellular carcinoma in noncirrhotic patients. A laparoscopic study of 92 cases in Taiwan. Cancer. 1984;54:1466-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Luo JC, Hwang SJ, Chang FY, Chu CW, Lai CR, Wang YJ, Lee PC, Tsay SH, Lee SD. Simple blood tests can predict compensated liver cirrhosis in patients with chronic hepatitis C. Hepatogastroenterology. 2002;49:478-481. [PubMed] |
| 23. | Schlichting P, Christensen E, Andersen PK, Fauerholdt L, Juhl E, Poulsen H, Tygstrup N. Prognostic factors in cirrhosis identified by Cox's regression model. Hepatology. 1983;3:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 116] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 272] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Nagamatsu H, Kumashiro R, Itano S, Matsugaki S, Sata M. Investigation of associating factors in exacerbation of liver damage after chemotherapy in patients with HBV-related HCC. Hepatol Res. 2003;26:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Christensen E, Schlichting P, Fauerholdt L, Gluud C, Andersen PK, Juhl E, Poulsen H, Tygstrup N. Prognostic value of Child-Turcotte criteria in medically treated cirrhosis. Hepatology. 1984;4:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 203] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Kao JH. Hepatitis B viral genotypes: clinical relevance and molecular characteristics. J Gastroenterol Hepatol. 2002;17:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 248] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Liu CJ, Kao JH, Chen PJ, Lai MY, Chen DS. Molecular epidemiology of hepatitis B viral serotypes and genotypes in taiwan. J Biomed Sci. 2002;9:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J Med Virol. 2004;72:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Grandjacques C, Pradat P, Stuyver L, Chevallier M, Chevallier P, Pichoud C, Maisonnas M, Trépo C, Zoulim F. Rapid detection of genotypes and mutations in the pre-core promoter and the pre-core region of hepatitis B virus genome: correlation with viral persistence and disease severity. J Hepatol. 2000;33:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Rodriguez-Frias F, Buti M, Jardi R, Cotrina M, Viladomiu L, Esteban R, Guardia J. Hepatitis B virus infection: precore mutants and its relation to viral genotypes and core mutations. Hepatology. 1995;22:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, Zhang HY, Lie A, Ngan R. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 332] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 34. | Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, Chan AT, Mok TS, Lee JJ, Leung TW. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |