INTRODUCTION
Gastric acid is essential for the sterilization of food and water and for digestion. The very low pH of gastric juice (pH~1) is the result of H+ and Cl- ion secretion (hydrochloric acid [HCl] production) by parietal cells in the oxyntic mucosa of the stomach. Each day, these cells secrete 1-2 L of HCl to achieve a concentration of 150-160 mmol/L in the stomach lumen. This acid concentration, which represents a very high accumulation of H+ in the lumen compared with parietal cell plasma, is facilitated by the activity of gastric H+,K+-ATPase in the apical membrane of the cell. K+ has a critical role in the activation of gastric H+,K+-ATPase and is essential for the functioning of the enzyme[1-3]. This review examines the central role of K+ in acid secretion and assesses how targeting this cation may provide novel ways of inhibiting gastric acid production.
GASTRIC H+,K+-ATPASE AND ACID SECRETION
Gastric H+,K+-ATPase is located in the apical membrane of the parietal cell and transports H+ into the parietal cell canaliculus in exchange for K+. The cations are exchanged in a 1:1 ratio, which maintains electroneutrality[4,5]. The enzyme is a member of the P-ATPase family that also includes Na+,K+-ATPase, Ca2+-ATPase and colonic H+,K+-ATPase[6-8]. Gastric H+, K+-ATPase shares many features, including structure and enzymatic mechanism, with other members of the family. A central feature of the P-ATPase family is that the energy for the translocation of ions is provided by ATP.
The amount of energy released on hydrolysis of an ATP molecule effectively limits the concentration gradient that can be created by a P-ATPase enzyme. The transport of ions is also constrained if their exchange generates a charge. As the translocation of H+ and K+ is electroneutral, gastric H+,K+-ATPase achieves a 3-4 million-fold ion concentration gradient (the difference in H+ concentration between plasma and parietal cell canaliculus), which is among the highest observed in the mammalian body.
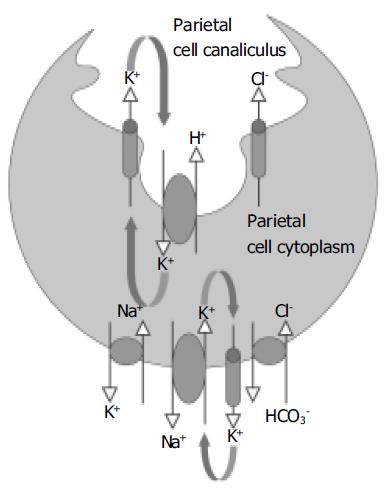
The process of acid secretion also involves the movement of other ions into and out of the parietal cell[1,9,10]. In the classical model of gastric acid secretion, we find that for each H+ ion that is transported into the canaliculus by the H+,K+-ATPase, the basolateral Cl-/HCO3- exchanger delivers a HCO3- molecule into the plasma and a Cl- ion into the cytosol (Figure 1)[11,12]. Cl- is secreted into the canaliculus via Cl- channels in the apical membrane of the parietal cell, and it is possible that more than one type of channel is involved, as is likely to be the case with K+. However, so far there is only evidence for the ClC-2 channel[13]. These Cl- channels allow Cl- to act as a counter ion for the K+ flux across the membrane, thereby balancing the charge and ensuring electroneutral HCl secretion.
Figure 1 A simplified model for the secretion of gastric acid by the parietal cell.
The H+,K+-ATPase located on the apical membrane of the parietal cell exchanges H+ for K+. K+ is recycled from the canaliculus into the cytoplasm by K+ channels in the apical membrane. The combined actions of K+ channels and enzymes on the basolateral membrane regulate cytoplasmic K+ levels. Cl- enters the cell cytoplasm via a Cl-/HCO3- exchanger and moves from the cytoplasm into the canaliculus via a Cl- channel (most likely ClC-2).
In common with most other cells, the level of K+ in the parietal cell is higher than that in the plasma. The higher intracellular K+ level is dependent on the activity of the Na+, K+-ATPase. This enzyme is located on the basolateral membrane of the cell where it exchanges intracellular Na+ for extracellular K+[14]. The level of K+ within the cell is also regulated by K+ channels that allow ion movement across the basolateral membrane. These channels have a particularly an important role in generating negative cell membrane potential.
ACTIVATION OF GASTRIC H+,K+-ATPASE BY K+
When a parietal cell is in a resting state, H+,K+-ATPase is localized to tubulovesicular elements within the cell[15]. The concentration of K+ in the tubulovesicular elements is low and their membranes are impermeable to K+. Consequently, the enzyme cannot be activated and transport H+ ions[1]. Stimulation of the parietal cell (e.g. by histamine) causes the tubulovesicular elements to fuse with the apical membrane of the cell[16,17]. The H+,K+-ATPase does not appear to undergo any chemical modification during activation of acid secretion but, as a result of the membrane fusion events, the enzyme is exposed to K+-containing luminal fluid and can start to exchange H+ for K+.
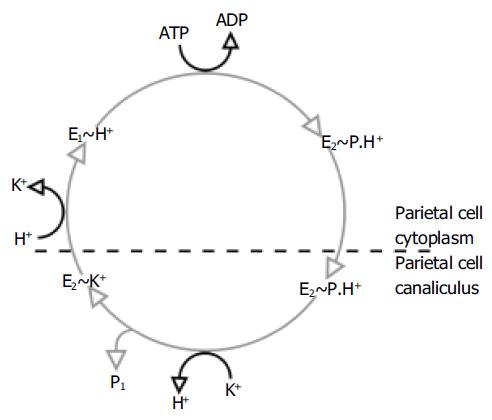
This process of ion exchange involves several steps and conformational changes in the 3D structure of the H+,K+-ATPase (Figure 2)[5]. The enzyme exists in two important conformational states. In one of these states (referred to as E1), the ionbinding site faces the parietal cell cytoplasm with high affinity for H+ and low affinity for K+; in the other state (E2), the ion-binding site faces the extracellular canaliculus with low affinity for H+ and high affinity for K+. It is likely that the shape of the K+ binding site or the path through which the ion accesses the binding site is different for the E1 and E2 forms[18], which may explain the relative affinity of the two forms for K+.
Figure 2 Post-Albers catalytic cycle of gastric H+,K+-ATPase.
In the E1 form, the enzyme takes up H+ and then converts into the E2 form with the hydrolysis of ATP. As well as providing the energy for the shift between these conformational states, the hydrolysis of ATP also results in the phosphorylation of the enzyme (a state referred to as E2P). The transformation to the E2 form causes the translocation of H+ from the parietal cell membrane into the canaliculus. Subsequently, the phosphorylated E2 form binds K+, which is required for the dephosphorylation of the H+,K+-ATPase.
During the cycling of the enzyme, K+ becomes temporarily occluded within the transmembrane segments and the cations no longer have free access to the cytoplasm or canaliculus. It is in this state that the cation causes dephosphorylation of the H+,K+-ATPase[19]. The mechanism for this process is not fully elucidated. It is likely that K+ does not stimulate dephos-phorylation of the phosphorylated intermediate directly, but acts by neutralizing the inhibitory effect of a negative charge in the membrane[20]. After dephosphorylation, the enzyme returns to its E1 form and releases K+ into the parietal cell cytoplasm.
Some investigators have reported that one H+ and one K+ are exchanged for each ATP hydrolyzed[21], while others have found that there is reciprocal exchange of two pairs of ions per ATP hydrolysis[22,23]. The hypothesis that one H+ is swapped for one K+ ion is supported by recent modeling work, which demonstrated that gastric H+,K+-ATPase has a single K+ binding site[24]. There are single ion binding sites in other P-type ATPases, such as yeast and plant H+-ATPase[25], which provides support for the existence of a single cation binding site in gastric H+,K+-ATPase. Moreover, it has been proposed that one single binding site could more easily explain the ability of the H+,K+-ATPase to transport H+ against a high concentration gradient[24].
K+ BINDING SITE IN GASTRIC H+,K+-ATPASE
Much work has been directed at elucidating the identity of the K+ binding site (or sites) and potential sites have been located within the transmembrane segments M4, M5, M6 and possibly M8 of the α-subunit of the enzyme[26-29]. In accordance with the physiological role and location of K+ (i.e. in the parietal cell canaliculus), the evidence suggests that the site is located towards the luminal face of the membrane domains[30]. When K+ occupies this binding site, it appears to affect the conformation of a large intracellular loop in which phosphorylation occurs[20]. Moreover, K+ is required for stabilization of a tight loop or ‘hairpin’ between M5 and M6[31]. This hairpin has a direct link with the phosphorylation domain on the intracellular loop that contains the ATP binding site, suggesting that it is involved in coupling ATP hydrolysis with cation transport[31].
As noted, recent work indicates that there is one high-affinity cation binding site in gastric H+,K+-ATPase[24]. The K+ binding site is formed by amino acids from M4, M5 and M6, with the ion being held in place by six oxygen atoms provided by these domains[24]. The presence of a negative charge within the pocket (at residue 820) is thought to be important to the functioning of the enzyme[24].
This understanding of the likely structure of the K+ site and its interaction with the phosphorylation domain has led to a theory on how K+ activates the enzyme. The negative charge in the ion-binding pocket may exert an indirect inhibitory effect on the phosphorylated intermediate form of the enzyme, thereby preventing its hydrolysis[20]. When the cation-binding pocket is occupied by K+, the negative charge is neutralized. A signal is then transferred to the nearby phosphorylation domain of the enzyme, possibly via a lysine amino acid residue, which results in enzyme dephosphorylation[32] and the subsequent translocation of K+ from the parietal cell canaliculus to the cytoplasm.
K+ SELECTIVITY OF THE H+,K+-ATPASE
The H+,K+-ATPase is highly selective for K+, although it can also be activated in vitro by NH4+[33,34]. The cation selectivity of the enzyme appears to be generated through interactions with the residues of the transmembrane segments of the α-subunit and the flanking loops that connect these transm-embrane domains[35]. The degree of K+ affinity, along with the ATPase activity of the gastric H+,K+-ATPase, is also influenced by a salt bridge from M5 to M6 that exists only when the enzyme is in the E2 form[24]. Importantly, this salt bridge only allows space for a single K+ binding site and so prevents the formation of another K+ binding site within the enzyme[24].
The β-subunit is also implicated in determining the K+ affinity of gastric H+,K+-ATPase[36,37], as shown in a study comparing the pig and rat gastric H+,K+-ATPase. The different K+ affinity of the enzymes from the two species was influenced by both the lipid matrix in which the enzymes were embedded and the identity of the β-subunit[37].
ROLE OF K+ CHANNELS IN K+ RECYCLING
At basal (i.e. unstimulated) levels of parietal cell activity, gastric juice consists mainly of NaCl, with only small amounts of K+ and H+. Stimulation of the parietal cell results in a sharp drop in pH. At a pH of approximately 1, the canaliculus of the parietal cell contains 150-160 mmol/L HCl[14]. Given the low concentration of K+ ions in the unstimulated state, achieving such a low pH would appear to be difficult through 1:1 exchange of H+ for K+. However, stimulation actually elevates K+ concentration (measured as KCl) in the gastric milieu (to 10-20 mmol/L KCl)[14]. Nevertheless, even at these levels there would be rapid K+ depletion without a mechanism for replenishing of K+ levels in the parietal cell canaliculus.
The K+ ions that are exchanged with H+ by the H+,K+-ATPase are provided by the parietal cell, while the Na+,K+-ATPase enzyme on the basolateral membrane accumulates K+ within the parietal cell. Theoretically, a K–Cl co-transporterd could supply the H+,K+-ATPase enzyme with K+, while also delivering Cl– into the parietal cell canaliculus. This does not however appear to be the case, as Cl- channels are present in the parietal cell apical membrane, and there is evidence that K+ is recycled and enters the parietal cell canaliculus via specific K+ channels[1].
To date, three different types of K+ channels that may contribute to K+ recycling have been identified in the apical membrane of the parietal cell. These K+ channels belong to one of the largest and most diverse group of ion channels in the body[38]. There are over 60 K+ channels, categorized according to whether they have 2, 4 or 6 transmembrane domains, and sharing a common feature of a pore-forming loop as part of the K+ selective filter. Associated with these pore-forming subunits are additional regulatory units. Although the subunits are not essential for the formation of the ionic pore, they are responsible for membrane targeting and functional properties of the channel. The identity of the subunits in a cell, along with the type of K+ channel they co-assemble with, determines the electrophysiological properties of that cell. In any given cell, there may be several types of channel and various subunits; some of the subunits may associate with more than one channel and so may result in channels with different functional attributes.
The K+ channel KCNQ1 (formerly known as KvLQT1) was found to co-localize with gastric H+,K+-ATPase and to be abundantly expressed in human and mouse gastric mucosa[39,40]. Electrophysiological assessment of the KCNQ1 channel confirmed it had sustained activity at low pH[39,40]. This is an essential property of ion channels involved in acid secretion, as they must be capable of functioning at a low pH. The subunit KCNE2 (and possibly KCNE3) appears to co-assemble with KCNQ1 to form a functional K+ channel in the apical membrane of parietal cells[40]. It is this subunit that is thought to determine the voltage dependence of KCNQ1 and its activation in response to extracellular acidification[40].
KCNQ1 has been proposed as an important K+ channel in the apical membrane, as gastric acid secretion was inhibited by the ‘specific’ KCNQ1 channel inhibitor, chromanol 293B[40]. However, it has since been suggested that chromanol 293B has an alternative, unidentified target in the parietal cell[41]. Nevertheless, the contribution of this channel to acid secretion is further supported by the fact that KCNQ1 gene disruption leads to a loss of acid secretion in knockout mice[42].
Several members of another type of K+ channel family, the inward rectifying K+ (Kir) family, have been shown to be expressed in rat gastric mucosa[43]. The Kir channels that were detected included Kir4.1, 4.2 and 7.1, although only Kir4.1 was found in parietal cells, where it was located at the apical region and appeared to co-localize with the β-subunit of H+,K+-ATPase.
Another member of the Kir family may also be involved in gastric acid secretion[41]. In rabbit gastric mucosa, high levels of Kir2.1 were detected along with lower levels of Kir4.1 and 7.1. Kir2.1 was found to be expressed in parietal cells from rabbit gastric mucosa and appeared to co-localize with H+,K+-ATPase and ClC-2 Cl- channels. The K+ channels were more likely to be open (i.e. allow K+ transit) when they were obtained from stimulated stomach than from resting stomach. A reduction in pH also tended to increase the likelihood that the channels would be open, which suggests that these channels are regulated in a similar fashion to ClC-2 Cl- channels. These findings, in a model that has been widely used to study the physiology of gastric acid secretion, suggest a role for Kir2.1 in K+ recycling. However, as the electrophysiological properties of the channel were studied in gastric vesicles, caution must be applied when extrapolating the results to the native environment of the cell[41]. As with the Kir4.1 channel, the Kir2.1 channel associates with four subunits to form a functional K+ channel.
Studies thus far have revealed a variety of potassium channels (i.e. KCNQ1, Kir2.1 and Kir4.1), albeit in different species, in the apical membrane of the parietal cell. All three channels have properties that are consistent with a role in K+ recycling. However, it is uncertain which of these channels, if any, plays the major role in K+ efflux and, as with the Cl– channel(s), a complete understanding of the K+ channel(s) involvement has yet to be achieved. Further study in both native tissues and transgenic animals may allow a more definite answer. A variety of K+ channels have also been identified on the basolateral membrane of parietal cells, each with distinctive properties[44]. Thus, it is not unreasonable to assume that more than one type of K+ channel in the apical membrane of the parietal cell may be involved in recycling the cation. Moreover, as noted previously, alongside this potential diversity of K+ channels, different subunits may exist in the same cell, which may affect the properties of the channels[45,46]. This raises the possibility of a variety of functional channels with subtly different electrophysiological properties, making elucidation of the relative contribution of different K+ channels extremely difficult. The present evidence indicates that more than one channel protein localizes to the apical region of the gland; as a result, the elimination of one channel may only lead to the upregulation of an alternative channel.
Caution must be applied in translating the findings in animal studies to man. For example, a single amino acid change can have a major influence on function. Thus, detailed studies need to determine the identity and composition of K+ channels in humans before it can be confirmed which channel or channels are important in apical membrane K+ flux. It also remains to be ascertained whether these channels are constitutively active or are regulated upon cell activation.
K+ AS A TARGET FOR BLOCKING GASTRIC ACID PRODUCTION
The importance of K+ in the production of gastric acid makes it a potential target for therapeutic intervention. One strategy is to block the K+ channels that are responsible for the flow of the ion across the parietal cell apical membrane, while an alternative pharmacological approach is to compete with K+ at the level of the gastric H+,K+-ATPase.
K+ channel blockers
The K+ channels in the apical membrane of the parietal cell represent a site for pharmacological modulation. The inhibition of gastric acid secretion by isolated gastric glands exposed to the ‘specific’ KCNQ1 K+ channel blocker, chromanol 293B, indicates the potential of such an approach[40]. Even if a K+ channel blocker did prevent H+, K+-ATPase activity, other challenges hinder the development of a therapeutic K+ channel blocker. The identity of the channel(s) involved in K+ recycling in the parietal cell will require further investigation, as will the possibility that more than one channel is involved in cation flux (which would require either several blockers or a drug that could inhibit a variety of channels). In addition to this potential problem, many of the identified ion channels can also be found in a variety of tissues (e.g. Kir4.1 is found on brain astrocytes[47] as well as in the apical membrane of parietal cells). This multi-organ distribution of channel proteins make organ-specific inhibitors a requirement, but a difficult target given the degree of cross-tissue homology exhibited by channel proteins.
Potassium-competitive acid blockers (P-CABs)
This group of mechanistically similar, developmental compounds has been identified as a potential therapeutic option for gastro-esophageal reflux disease (GERD) and other acid-related disorders[48]. They inhibit gastric H+, K+-ATPase by binding ionically to the enzyme and thus prevent activation by the K+ cation. It is likely that P-CABs bind at or near the K+ binding site and so prevent access of the cation to the site.
The early developmental compound, SCH28080, exemplifies the mechanism of action of P-CABs. This agent inhibited gastric acid production in healthy volunteers[49] and, although clinical development was not continued, it has been used extensively to explore the mechanisms of inhibition of gastric H+,K+-ATPase.
The large size of SCH28080 compared with K+ ions suggests that the ion-binding site and inhibitor-binding site are not identical. Furthermore, mutational analysis of the gastric H+,K+-ATPase suggests that there are separate binding sites for SCH28080 and K+. For example, mutations of several amino acid residues in the membrane domains reduce affinity for SCH28080 but have no effect on K+ affinity[50,51]. Mutational analysis also indicates that the binding site of SCH28080 is closer to the luminal surface of the parietal cell than the ion-binding site[18].
SCH28080 gains access to its binding site and competes with K+ when the gastric H+,K+-ATPase is in the phosphorylated E2 form[52,53]. When the P-CAB binds to the H+,K+-ATPase, it stabilizes the enzyme in the E2 conformation and, thereby, prevents the movement of H+ ions into the parietal cell canaliculus. Mutational data suggest that SCH28080 binds near the loop between M5 and M6, and at the luminal end of M6, about two helical turns away from the ion-binding site[18]. Homology modeling has suggested that SCH28080 interacts with residues in the M1–M6 domains[54], and, more specifically, it docks in a cavity formed by the M1, M4, M5, M6 and M8 transmembrane segments and by loops formed by M5/M6, M7/M8 and M9/M10[55]. This specificity was also demonstrated by another P-CAB, SPI447 in the same study[55]. A P-CAB molecule cannot occupy its binding pocket when the enzyme is in the E1 form, due to rearrangement of the loop between M3 and M4, which alters the shape of the P-CAB binding cavity[55].
Inhibition of gastric acid production by P-CABs
The principle that P-CABs inhibit gastric H+,K+-ATPase was shown by studies with the experimental agents BY841[56], SCH28080[49], SK&F 96067[57], and SPI-447[58]. All P-CABs inhibit the gastric H+,K+-ATPase by competing with K+[57-60], and their physico-chemical properties allow them to concentrate preferentially in highly acidic environments. Clinical develop-ment of the class is continuing with AZD0865, CS-526 (R-105266), revaprazan (YH1885) and soraprazan (BY359). Animal and early clinical studies have demonstrated that P-CABs are highly selective for gastric H+,K+-ATPase and inhibit gastric acid secretion with a fast onset of action[57,58,61-68]. Treatments that provide faster onset of effect and increased duration of action would offer improvement for patients with GERD and other acid-related disorders. An interesting observation is that the gastric isoform of the H+,K+-ATPase has been identified in only in two organs: the stomach and the kidney. Drugs targeted directly at this protein have shown no adverse effects on renal function, in either animal models or in humans following prolonged use. This is likely due to the relatively high pH in the kidney, which serves to preclude accumulation of these agents. Publication of ongoing studies investigating the efficacy of P-CABs in the treatment of GERD and other acid-related disorders are awaited.
CONCLUSION
In summary, we continue to gain insights into the role of K+ in gastric H+,K+-ATPase function. This cation is not only the counterpart for H+ that allows the generation of a highly acidic environment in the parietal cell canaliculus, but it is also an essential ion for the functioning of the enzyme itself. Investigations into the structure of the gastric H+,K+-ATPase indicate that the K+ binding site is formed by amino acids from the M4, M5 and M6 domains of the enzyme. With the development of transgenic animals and new molecular markers, we are now only beginning to examine some of the subtle mechanisms by which K+ induces the conformational changes that result in ion translocation.
The dependence of gastric H+,K+-ATPase on K+ requires that relatively high levels of the cation are available in the parietal cell canaliculus. There is evidence that K+ is recycled and enters the parietal cell canaliculus via specific K+ channels. Three potential K+ channels (KCNQ1, Kir2.1, and Kir4.1) that may contribute to K+ recycling have been identified in the apical membrane of the parietal cell. The essential contribution of K+ to gastric acid secretion makes K+ channels attractive candidates for approaches to block acid production.
The specific KCNQ1 K+ channel blocker, chromanol 293B, suggests the potential of such an approach. However, when the apical K+ channels are inhibited by barium, the gastric H+,K+-ATPase continues to function. Despite the theoretical attractiveness of this approach, it is uncertain which of these channels, if any, plays the major part in cation recycling across the cell membrane and it is possible that more than one channel is involved. Moreover, homologous (or identical) K+ channels can occur in different tissues, thereby making the development of an organ-specific channel blocker a challenging task. As research continues into the molecular identification of the apical K+ channel(s) and their occurrence in other tissues, we will be able to assess whether these proteins are viable targets for inhibition of gastric acid secretion.
A group of mechanistically similar developmental compounds, the P-CAB class has been identified as a potential option for acid suppression therapy. P-CABs compete with K+ at the level of the enzyme to inhibit acid production; the binding site of these agents appears distinct from the likely pocket that K+ occupies. When the P-CAB occupies its binding site, it prevents K+ from binding to and activating the enzyme. Animal and early clinical studies demonstrate that P-CABs are highly selective for gastric H+,K+-ATPase and inhibit gastric acid secretion with a fast onset of effect. Such treatments that offer the potential of a fast onset of effect and a long duration of action may provide significant benefits to patients with GERD and other acid-related disorders.
Clearly, by continuing to define the role of K+ in gastric acid secretion, we will advance the development of novel approaches to regulate the production of gastric acid.