Published online Sep 7, 2005. doi: 10.3748/wjg.v11.i33.5235
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: September 7, 2005
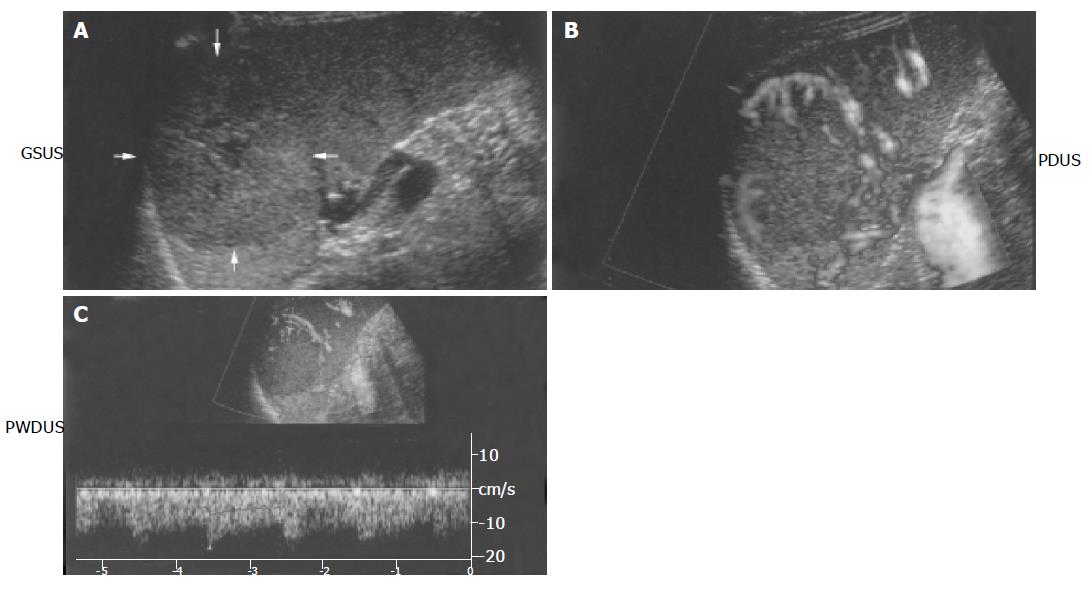
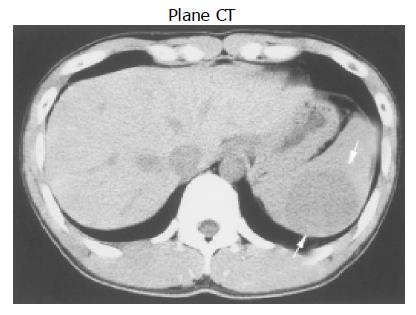
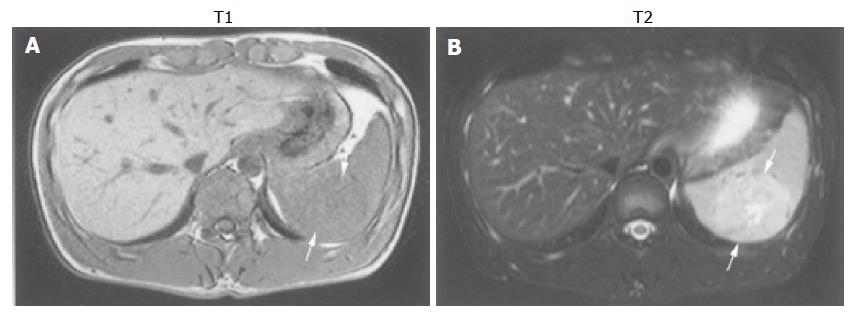
We present the gray-scale ultrasonography (GSUS), power Doppler ultrasonography (PDUS), abdominal computed tomography (CT), and magnetic resonance imaging (MRI) findings for a case of splenic hamartoma in a 27-year-old man, showing a φ 50 mm homogeneous, iso- and hypo-echoic splenic mass with evidence of a small plural cystic lesion. This splenic hamartoma showed increased vascularity on power Doppler sonograms. PDUS showed multiple circular blood flow signals inside the mass (i.e. a basket pattern), which was consistent with the small plural cystic lesion shown by GSUS. Spectral analysis also confirmed arterial and venous flow. CT scans showed that the mass had low-density relative to the normal spleen and MRI showed that the mass was isodense, relative to the normal spleen. Therefore, CT and MRI are not useful for the diagnosis of splenic hamartoma. Ultrasonography can be used to diagnose splenic hamartoma without administration of a contrast material and therefore is an indispensable method for the diagnosis of splenic hamartoma.
- Citation: Nakanishi S, Shiraki K, Yamamoto K, Nakano T, Koyama M, Yano T, Sanda T, Tamaki H, Hirano T, Fukudome K, Ishihara A. Basket pattern blood flow signals discovered in a case of splenic hamartoma by power Doppler ultrasonography. World J Gastroenterol 2005; 11(33): 5235-5238
- URL: https://www.wjgnet.com/1007-9327/full/v11/i33/5235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i33.5235
Splenic hamartoma is a rare benign tumor with a mortality rate of 0.13%[1]. Since the first description by Rokitansky in 1 861[2], more than 100 cases have been reported. However, hamartoma images have rarely been reported. On sonograms, most splenic hamartomas are a homogeneous and solid mass, with varying echogenicity relative to the normal splenic parenchyma[3-6]. Also, the power Doppler ultrasonography (PDUS) findings of splenic hamartomas show increased vascularity[7]. To our knowledge, PDUS findings, computed tomography (CT), magnetic resonance imaging (MRI) features, and pathologic findings of a splenic hamartoma have not been reported.
The patient was an asymptomatic 27-year-old man. Routine physical examination and blood test had no remarkable finding.
Sonographic examination of the patient’s spleen was performed using the Aplio80 (Toshiba SSA-770A) equipped with a 4.5-MHz convex-array transducer. Gray-scale ultras-onography (GSUS) showed a φ 4.3 cm×4.7 cm hypo-echoic and iso-echoic (i.e. mixed pattern) solid mass (Figure 1A) at the middle pole of the spleen. The mass was homogeneous with evidence of cystic changes in the center, but without calcification. Sonography also showed a clear and smooth margin, and PDUS showed multiple circular blood flow signals inside the mass (i.e. basket pattern) (Figure 1B). Arterial and venous flow was confirmed by spectral analysis (Figure 1C) and the peak systolic velocity of the arterial flow noted in the mass was 16.4 cm/s with a resistance index of 0.38 and a pulsatility index of 0.52.
Abdominal CT scan before administration of a contrast material revealed a low-density mass (Figure 2). During the arterial phase of contrast-enhanced dynamic CT, the mass was enhanced earlier than the normal splenic parenchyma. During the delayed phase, the intensity of the mass was the same as that of the normal splenic parenchyma.
MRI showed that the mass in the spleen was iso-intense relative to the normal spleen on T1-weighed images and mild hyper-intense on T2-weighed images (Figure 3). During the arterial phase of contrast-enhanced dynamic MRI, the mass was enhanced earlier than the normal splenic parenchyma. During the delayed phase, the mass was mild hyperintense relative to the normal splenic parenchyma. These findings were suggestive of hemangioma, metastatic tumor, malignant lymphoma, angiosarcoma or hamartoma.
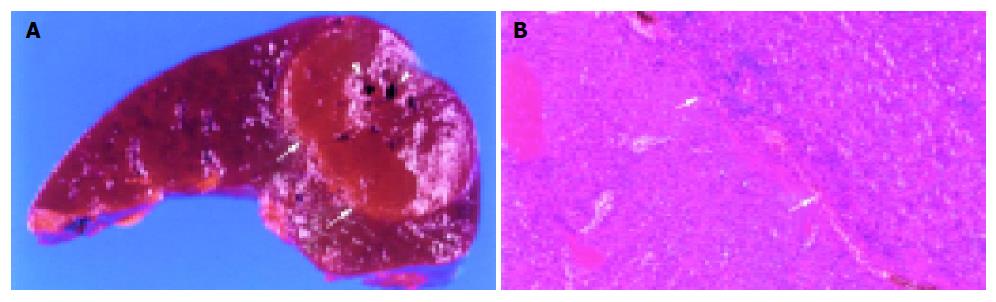
A splenectomy was performed because a malignant splenic neoplasm or metastasis could not be ruled out, as it could prevent the mass from spontaneous rupture. Histologic examination of the lesions revealed dilated vessels and congestion. The border of the mass and normal splenic parenchyma was smooth and clear (Figure 4A). The mass was composed of red pulp and lacked fibrous trabeculae and white pulp. The normal splenic parenchyma was compressed, and no capsule was found (Figure 4B).
The final pathologic diagnosis was a splenic hamartoma of the red pulp.
Splenic hamartoma is prevalent in either sex and has been reported in all age groups. However, it was reported that 50% of pediatric patients with a splenic hamartoma have clinical manifestations such as pancytopenia, anemia or thrombocytopenia[3]. Spontaneous rupture of the hamartoma with acute abdominal pain in adults has also been reported[2,8]. Here we present a case who was asymptomatic, and the splenic tumor was coincidentally discovered, during a complete medical checkup.
Splenic hamartoma is a rare benign tumor. Wan et al[9], have reported the sonographic findings in 53 cases of verified focal splenic lesions, such as cysts, infarcts, abscess, and hemangioma, spontaneous rupture, tuberculosis, lymp-hangioma, and hamartoma.
On gray-scale sonograms, most splenic hamartomas are solid and homogeneous masses with various echogenicities relative to the normal splenic parenchyma[3-6]. However, sonographic echogenicity indicates that splenic hamartoma is usually hypo-echoic[7,10], and occasionally heterogeneous and may exhibit cystic changes or calcification[4,11]. In our case, splenic hamartoma showed some solid and homogeneous characteristics via the gray-scale sonographic findings. However, internal echogenicity of the mass showed mixed pattern echoes containing a cystic area, but the posterior echo was only slightly enhanced. Gray-scale sonographic findings on tissue harmonic imaging (THI) have not been reported regarding the boundary echo of a splenic hamartoma. The gray scale of the boundary echoes shows a clear, smooth, and hyper-echoic rim. THI improves the resolution, because the high-condition wave beam is sharper than a standard wave beam. Also, THI is useful for improving the resolving power of the tumor. Splenic hamartomas are usually detected as hypo-echoic tumors, but in our case the echogenicity of the splenic hamartoma was iso-echoic and hypo-echoic (i.e. mixed pattern) on THI for gray-scale sonography. Splenic hamartomas are usually detected as hypervascular tumors by arteriography. Angiograms of the mass on power Doppler imaging (PDI) show tumor vessels with aneurysmal dilatation, arterio-venous shunts, vascular lakes, and tumor blush, presenting a typically malignant vascular pattern[12]. This is different from those on color Doppler image (CDI), because there is almost no dependence on the angle. Therefore, PDI exhibits excellent sensitivity compared to CDI. Tang et al[7], have reported color Doppler sonographic findings in splenic hamartoma, and found that blood flow is concentrated around the mass as multiple radial blood flow signals. However, we found that blood flow was concentrated around the mass as surrounding blood flow signals coincident to the boundary echo and as multiple circular blood flow signals. Histologic analysis following splenectomy showed multiple vessels consistent with the PDUS findings.
Contrast-enhanced abdominal CT scans revealed that splenic hamartoma is iso-dense relative to the normal splenic parenchyma, before and after administration of intravenous contrast material[7,11]. The mass in our patient was iso-dense during the delay phase of contrast-enhanced dynamic CT, but had a low density relative to the normal splenic parenchyma, before administration of the intravenous contrast material. Also during the artery phase of contrast-enhanced dynamic CT, the mass had a high density relative to the normal splenic parenchyma.
Hamartomas of the spleen usually are iso-intense on T1-weighed MR images and hyper-intense on T2-weighed images[6,7] and the same was true for our case. However, Fernandez-Canton et al[13], described that a histologically proven case presents a small (2.5 cm) focal mass, which is iso-intense to the splenic parenchyma on T1-weighed images and hypo-intense on turbo-spin-echo T2 and short T1 inversion recovery images. In addition, Chevallier et al[10], reported that a fibrous-type splenic hamartoma measuring 7 cm in diameter that MRI showed is hyper-intense on T1-weighed MRI and hypo-intense on T2-weighed MRI.
Splenic hamartoma shows expansive growth and compresses the surrounding splenic tissue without a true capsule. Two histologic types of splenic hamartoma have been described[1]: the white-pulp (lymphoid) type and the red-pulp (pulposal) type. The white-pulp type is entirely composed of lymphoid tissue, whereas the red-pulp type defined by the Armed Forces Institute of Pathology as a hamartoma in 1995[14] is composed of sinuses and is histologically similar to that of normal splenic red pulp. Hamartomas are of the red-pulp type are the most reported cases, as was the case for our patient’s splenic hamartoma.
Sonography permits differential diagnosis of tumor of the spleen. A splenic hemangioma is hyper-echoic on gray-scale sonograms[5,15] and color Doppler sonograms show few color echoes similar to the findings in hepatic hemangiomas. Color Doppler sonograms show an avascular pattern of metastatic tumors and malignant lymphoma of the spleen[5]. Sonograms of angiosarcomas show multiple hyper-echoic masses[16] and color Doppler sonograms show a fluctuating low-velocity flow pattern[17].
Since splenic hamartomas do not always exhibit hype-rvascularity[4], it should be included in the differential diagnosis of splenic tumors in which color Doppler sonograms show abundant blood flow. However, splenic hamartomas are usually hypervascular tumors. The circular signals on power Doppler sonograms (basket pattern) in our case are consistent with the histologic findings.
In conclusion, abdominal CT and MRI require the admi-nistration of a contrast material to make an exact diagnosis. However, PDUS does not require the administration of a contrast material. Furthermore, PDUS does not affect the body. Therefore, the results of PDUS are equivalent to abdominal CT and MRI.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Silverman ML, Livolsi VA. Splenic hamartoma. Am J Clin Pathol. 1978;70:224-229. [PubMed] |
| 2. | Morgenstern L, McCafferty L, Rosenberg J, Michel SL. Hamartomas of the spleen. Arch Surg. 1984;119:1291-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Iozzo RV, Haas JE, Chard RL. Symptomatic splenic hemartoma: a report of two cases and review of the literature. Pediatrics. 1980;66:261-265. [PubMed] |
| 4. | Komaki S, Gombas OF. Angiographic demonstration of a calcified splenic hamartoma. Radiology. 1976;121:77-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Goerg C, Schwerk WB. Color Doppler imaging of focal splenic masses. Eur J Radiol. 1994;18:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Ohtomo K, Fukuda H, Mori K, Minami M, Itai Y, Inoue Y. CT and MR appearances of splenic hamartoma. J Comput Assist Tomogr. 1992;16:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Tang S, Shimizu T, Kikuchi Y, Shinya S, Kishimoto R, Fujioka Y, Miyasaka K. Color Doppler sonographic findings in splenic hamartoma. J Clin Ultrasound. 2000;28:249-253. [PubMed] [DOI] [Full Text] |
| 8. | Ferguson ER, Sardi A, Beckman EN. Spontaneous rupture of splenic hamartoma. J La State Med Soc. 1993;145:48-52. [PubMed] |
| 9. | Wan YL, Cheung YC, Lui KW, Tseng JH, Lee TY. Ultrasonographic findings and differentiation of benign and malignant focal splenic lesions. Postgrad Med J. 2000;76:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Chevallier P, Guzman E, Fabiani P, Dib M, Oddo F, Padovani B. [Fibrous splenic hamartoma: imaging features]. J Radiol. 1999;80:1668-1671. [PubMed] |
| 11. | Zissin R, Lishner M, Rathaus V. Case report: unusual presentation of splenic hamartoma; computed tomography and ultrasonic findings. Clin Radiol. 1992;45:410-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kishikawa T, Numaguchi Y, Watanabe K, Matsuura K. Angiographic diagnosis of benign and malignant splenic tumors. AJR Am J Roentgenol. 1978;130:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Fernandez-Canton G, Capelastegui A, Merino A, Astigarraga E, Larena JA, Diaz-Otazu R. Atypical MRI presentation of a small splenic hamartoma. Eur Radiol. 1999;9:883-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Warnke RA, Weiss LM, Chan JK. Tumors of the lymph nodes and spleen. Atlas of tumor pathology. Series 3, Fascicle 14. Washington, DC: Armed Forces Institute of Pathology 1995; PMC1909076. |
| 15. | Niizawa M, Ishida H, Morikawa P, Naganuma H, Masamune O. Color Doppler sonography in a case of splenic hemangioma: value of compressing the tumor. AJR Am J Roentgenol. 1991;157:965-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Ha HK, Kim HH, Kim BK, Han JK, Choi BI. Primary angiosarcoma of the spleen. CT and MR imaging. Acta Radiol. 1994;35:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Aytaç S, Fitoz S, Atasoy C, Kuzu I, Cinar K, Erden I. Multimodality demonstration of primary splenic angiosarcoma. J Clin Ultrasound. 1999;27:92-95. [PubMed] [DOI] [Full Text] |