Published online Sep 7, 2005. doi: 10.3748/wjg.v11.i33.5199
Revised: February 15, 2005
Accepted: February 18, 2005
Published online: September 7, 2005
AIM: As tumor markers for pancreatic carcinoma, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 have been used, but the sensitivity and specificity are not enough for the diagnosis of pancreatic carcinoma.
METHODS: A novel serum tumor marker, RCAS1, was compared with two conventional serum tumor markers, CEA (highly specific for pancreatic cancer) and CA 19-9 (highly sensitive for pancreatic cancer), in 48 patients with pancreatic exocrine tumors.
RESULTS: When the diagnosis of benign or malignant conditions was examined by one tumor marker, the sensitivity of RCAS1 alone (55%) was higher than that of CEA alone (27%) and the specificity of RCAS1 alone (92%) was greater than that of CA19-9 alone (78%). When examined by a combination of two markers, the sensitivity of a combination of RCAS1 and CA19-9 (95%) was superior to those of CA19-9 alone (78%), RCAS1 alone (55%, P = 0.002), CEA alone (27%) (P<0.001), RCAS1 and CEA (59%) and CA19-9 and CEA (82%).
CONCLUSION: These results suggest that the combination of RCAS1 and CA19-9 is highly sensitive for pancreatic carcinoma.
- Citation: Yamaguchi K, Enjoji M, Nakashima M, Nakamuta M, Watanabe T, Tanaka M. Novel serum tumor marker, RCAS1, in pancreatic diseases. World J Gastroenterol 2005; 11(33): 5199-5202
- URL: https://www.wjgnet.com/1007-9327/full/v11/i33/5199.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i33.5199
RCAS1 (receptor-binding cancer antigen expressed on SiSo cells) is a novel tumor-associated antigen expressed in malignant conditions of various organs. A human cancer cell line, SiSo, was established from a patient affected with uterine cervical adenocarcinoma[1]. The mAb 22-1-1 was manufactured from mice immunized with SiSo cells. Then a cDNA encoding the antigen 22-1-1 was isolated and named RCAS1[2]. The predicted amino acid sequences of RCAS1 (213 aa) possess an N-terminal transmembrane region and a coiled-coil structure in the C-terminal portion, indicating that RCAS1 is a type II membrane protein and is able to form oligomers through the coiled-coil structure.
RCAS1 was found to be identical to EBA G9 (estrogen receptor-binding fragment-associated gene), which was initially identified by cloning of CpG binding sites in the human genome as estrogen responsive gene and mapped to chromosome 8q23. RCAS1 expresses a pattern different from other known tumor-associated antigens, such as YH206, GA733, CA125, CEA, and sialyl Le molecules. Immunohistochemical studies have reported the presence of RCAS1 in malignant diseases of several organs including the uterus[3], endometrium[4], ovary, skin[5], breast[6,7], liver[8], gallbladder[9], stomach[10], lymphocytes[11], and lung[12] as well as pancreas[13]. Previous studies have also indicated that RCAS1 acts as a ligand for a putative receptor present on various human cells including T, B, and NK cells and RCAS1 inhibits in vitro growth of receptor-expressing cells and induces apoptosis[14]. It is suggested that tumor cells might evade host immune surveillance by expression of RCAS1.
Recently, soluble RCAS1 was also detected by ELISA from the culture supernatant of a human cell line derived from bile duct carcinoma[15], suggesting a possible role of RCAS1 as a diagnostic or prognostic tumor marker. There are few reports on serum levels of RCAS1 in patients with pancreatic diseases. In this series, clinical implication of serum RCAS1 was examined by comparing with conventional tumor markers, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9, in 48 patients with pancreatic tumors.
This series consisted of 48 patients with pancreatic exocrine neoplasms and 23 with benign inflammatory pancreato-biliary diseases (control group). Out of the 71 patients, 35 were men and 36 women, and their age ranged from 18 to 89 years with a mean of 61.9±12.5 years. The 23 patients in the control group consisted of 18 with benign inflammatory biliary diseases (four cholecystolithiasis, four cholangitis, four benign gallbladder polyp, three hepatolithiasis, two adenomyomatosis of the gallbladder and one choledocholithiasis) and five with benign pancreatic diseases (four chronic pancreatitis, one pancreatolithiasis). The 48 patients with pancreatic neoplasms included 30 patients in the preoperative state of benign or malignant diseases (10 intraductal papillary-mucinous adenoma, 20 pancreatic carcinoma) and 18 patients in the postoperative state (3 intraductal papillary-mucinous adenoma, 4 intraductal papillary-mucinous adenocarcinoma and 11 pancreatic adenocarcinoma). Of the 15 postoperative patients with pancreatic carcinoma, 2 had unequivocal recurrence as judged by clinical findings including imagings, and the other 13 had no evidence of recurrence. In total, 22 patients were therefore considered to have pancreatic carcinoma. Serum levels of RCAS1, CEA, and CA19-9 were examined in all these 71 patients.
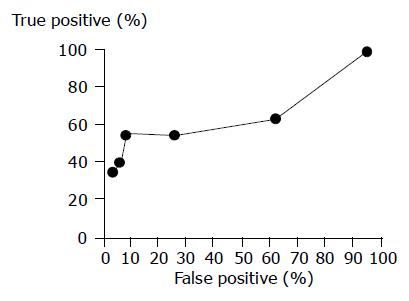
Peripheral blood was obtained in the early morning for the examination of RCAS1, CEA, and CA19-9. Serum RCAS1 level was measured using ELISA kit, which was kindly donated from Medical and Biological Laboratories Co., Ltd, Nagoya, Japan. The RCAS1 ELISA kit measures RCAS1 by sandwich ELISA. The assay used mAbs against RCAS1. The serum obtained was pre-treated with neuraminidase. Pretreated samples to be measured or standards were incubated in the cells coated with anti-human RCAS1 mAb; 22-1-1. After washing, a biotin-conjugated anti-human RCAS1 mAb was added into the microwell and incubated. After another washing, the streptavidin-peroxidase was added into the microwell and incubated. After another washing, the peroxidase substrate premixed with chromogen was added and allowed to incubate for an additional period of time. An acid solution was then added to each microwell to terminate the enzyme reaction and to stabilize the developed color. The optical density (A) of each microwell was then measured at 450 nm using a microplate reader. The concentration of RCAS1 was calibrated from a dose-response curve based on reference standards. Cut-off level was set at 20 U/mL by constructing a receiver operating characteristic curve (Figure 1). Serum CEA and CA19-9 levels were measured in Clinical Laboratory of Kyushu University Hospital. Their cut-off levels were 2.5 ng/mL and 37.4 IU/mL, respectively.
Clinical stages of malignant pancreatic diseases were determined by the Classification of Pancreatic Carcinoma from Japan Pancreas Society (First English Edition)[16]. Out of the 22 patients with primary or metastatic pancreatic adenocarcinoma, 1 was in stage I, 1 in stage III, 3 in stage IVa and the other 17 in stage IVb. Four of the twenty-two patients underwent pancreatic resection; three pylorus preserving pancreato-duodenectomy and another distal pancreatectomy.
Informed consent was obtained from each patient. The protocol was submitted to and was preapproved by the Senior Staff Committee of the Department.
Values were expressed as mean±SD. Mean values were measured by the Student’s t test and the distribution of patients was measured by the χ2 test. Sensitivity, specificity, positive predictive value, negative predictive value, and efficiency were measured. P<0.05 was considered as statistically significant.
Serum levels of RCAS1, CEA, CA19-9 in 26 patients with benign inflammatory bilio-pancreatic diseases were 12.1±1.1 U/mL, 1.1±0.1 ng/mL and 30.5±6.6 U/mL; 10 with adenoma of the pancreas (intraductal papillary-mucinous adenoma) 14.1±2.9, 1.2±0.2, and 13.5±4.4; and 22 with adenocarcinoma of the pancreas 113.0±70.8, 4.8±2.3, and 9 947.9±6 814.1, respectively. There were no statistically significant differences among the RCAS1, CEA, and CA19-9 values of the three conditions.
Data concerning the diagnosis of benign and malignant conditions are shown in Table 1. When comparing by one tumor marker, the sensitivity was highest in CA19-9 (73%), while the specificity was highest in CEA (94%). The total efficacy was highest in RCAS1 (78%). When two tumor markers were joined, the sensitivity was highest by a combination of RCAS1 and CA19-9, the value being 95%, whereas the specificity was highest by a combination of RCAS1 and CEA (86%). The total efficiency was highest by a combination of RCAS1 and CA19-9 (79%).
| RCAS1 (%) | CEA (%) | CA19-9 (%) | RCAS1+CEA (%) | RCAS1+CA19-9 (%) | CA19-9+CEA (%) | |||||||
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | |
| Malignant | 12 | 10 | 6 | 16 | 16 | 6 | 13 | 9 | 21 | 1 | 18 | 4 |
| Benign | 3 | 33 | 2 | 34 | 8 | 28 | 5 | 31 | 11 | 25 | 9 | 27 |
| Sensitivity | 553 | 27b2 | 731 | 592 | 95b3 | 82d | ||||||
| Specificity | 92 | 94 | 78 | 86 | 69 | 75 | ||||||
| Positive predictive value | 80 | 75 | 67 | 72 | 66 | 67 | ||||||
| Negative predictive value | 78 | 68 | 82 | 78 | 96 | 87 | ||||||
| Efficiency | 78 | 69 | 76 | 76 | 79 | 78 | ||||||
The positivity of the three markers increased by the progression of tumors (Table 2).
| Stage | RCAS1 | CEA | CA19-9 | RCAS1+CEA | RCAS1+CA19-9 | CA19-9+CEA | ||||||
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | |
| 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 3 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| 4A | 0 | 3 | 0 | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 3 | 0 |
| 4B | 11 | 6 | 6 | 11 | 12 | 5 | 12 | 5 | 16 | 1 | 14 | 3 |
The sensitivity of prediction of unresectability was highest by CA19-9 alone (71%), while the specificity was highest by CEA alone (100%, Table 3). When combination assay was done, the sensitivity was highest by RCAS1 and CA19-9 (94%), while the specificity was highest by RCAS1 and CEA (80%).
| Unresectability | RCAS1 (%) | CEA (%) | CA19-9 (%) | RCAS1+CEA (%) | RCAS1+CA19-9 (%) | CA19-9+CEA (%) | ||||||
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | |
| Yes | 11 | 6 | 6 | 11 | 12 | 5 | 12 | 5 | 16 | 1 | 14 | 3 |
| No | 1 | 4 | 0 | 5 | 4 | 1 | 1 | 4 | 5 | 0 | 4 | 1 |
| Sensitivity | 65 | 35123 | 712 | 713 | 94b | 821 | ||||||
| Specificity | 80 | 1004 | 20 | 80 | 0 | 20 | ||||||
| Positive predictive value | 92 | 100 | 75 | 92 | 76 | 78 | ||||||
| Negative predictive value | 40 | 31 | 17 | 44 | 0 | 25 | ||||||
| Efficiency | 68 | 50 | 59 | 73 | 73 | 68 | ||||||
Data are present in Table 4. When examined by one tumor marker, RCAS1 alone and CA19-9 alone showed a high sensitivity and specificity. When two tumor markers were combined, a combination of RCAS1 and CA19-9 showed a high sensitivity (100%) and high specificity (92%).
| Recurrence | RCAS1 (%) | CEA (%) | CA19-9 (%) | RCAS1+CEA (%) | RCAS1+CA19-9 (%) | CA19-9+CEA (%) | ||||||
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | |
| Yes | 2 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| No | 1 | 12 | 2 | 11 | 1 | 12 | 3 | 10 | 1 | 12 | 3 | 10 |
| Sensitivity | 100 | 50 | 100 | 100 | 100 | 100 | ||||||
| Specificity | 92 | 85 | 92 | 77 | 92 | 77 | ||||||
| Positive predictive value | 67 | 33 | 67 | 40 | 67 | 40 | ||||||
| Negative predictive value | 100 | 92 | 100 | 100 | 100 | 100 | ||||||
| Efficiency | 93 | 80 | 93 | 80 | 93 | 80 | ||||||
CA19-9 (a highly sensitive marker for pancreatic carcinoma) and CEA (a highly specific marker for pancreatic carcinoma) have been used as serum tumor markers for pancreatic diseases. Usefulness of RCAS1, as a tumor marker, was studied in 48 patients with pancreatic tumors. The sensitivity of RCAS1 alone for pancreatic carcinoma was 55%, which was higher than 27% of CEA alone and lower than 73% of CA19-9 alone. When combination assay was done, the sensitivity of a combination of RCAS1 and CA19-9 was highest, the value being 95%. The specificity of RCAS1 alone for pancreatic carcinoma was 92%, which was lower than 94% of CEA alone and higher than 78% of CA19-9 alone. The specificity by a combination of RCAS1 and CA19-9 was 69%. Combination assay of RCAS1 and CA19-9 was the most powerful marker for the detection of pancreatic carcinoma.
Clinical differentiation of benign inflammatory disease and malignant disease is important as well as that of benign neoplasm and malignant conditions. Mean serum levels of RCAS1, CEA, and CA19-9 in pancreatic carcinoma were higher than those of benign inflammatory diseases and pancreatic adenoma. The values in benign inflammatory diseases and adenoma were not different from each other. Therefore, although the differentiation of benign inflammatory diseases and adenoma was difficult by serum tumor markers alone, that of benign diseases and malignant conditions may be possible. Clinical diagnosis of pancreatic diseases should be made by clinical findings and imaging with the aid of serum tumor markers.
Originally, RCAS1 was suspected to be elevated only at advanced stage of pancreatic carcinoma, because RCAS1 is related with immune escape from the host immune and with carcinoma invasion. The specificity of RCAS1 for the presence of pancreatic carcinoma was expected to be high and the sensitivity was suspected to be low. The sensitivity of RCAS1 alone for malignant condition was 55%, which was higher than 27% by CEA alone, and the specificity of RCAS1 alone was 92%, higher than 78% of CA19-9 alone. Combination assay of RCAS1 and CA19-9 showed the highest sensitivity of 95%, although the specificity was 69%. The sensitivity of tumor marker is important to decrease the missing of pancreatic carcinoma and the specificity is also important to decrease the over diagnosis. Thus, combination assay of CA19-9 and RCAS1 is recommended for the detection of pancreatic carcinoma.
It is true that the surgical decision making is made on imaging or macroscopic findings, but the prediction of resectability by serum tumor marker is of great value for surgeons. The sensitivity of prediction of unresectability was 94% by a combination of RCAS1 and CA19-9 and the specificity was 80%. These findings indicate that elevation of serum RCAS1 and CA19-9 may be suggestive of unresectable pancreatic carcinoma, although the number of patients examined was so small, that the data obtained was not conclusive.
One of the purposes of serum tumor marker is to predict clinical outcome of patients with pancreatic carcinoma. Recent immunohistochemical reports suggest that RCAS1 expression is related to early stage of carcinogenesis[7], poor differentiation[4], progression of the disease[5,10], and poor prognosis of cancer[9,13,17]. In this series, clinical follow-up was so short that we could not yield data concerning the clinical outcome. Further examination using long-term follow-up is necessary to evaluate predictability of RCAS1.
Although this series is a preliminary report on serum RCAS1 in pancreatic diseases, combination assay of RCAS1 and CA19-9 showed 95% sensitivity for pancreatic carcinoma, suggesting usefulness of this combination as a checking-up marker for pancreatic carcinoma.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Sonoda K, Nakashima M, Kaku T, Kamura T, Nakano H, Watanabe T. A novel tumor-associated antigen expressed in human uterine and ovarian carcinomas. Cancer. 1996;77:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Nakashima M, Sonoda K, Watanabe T. Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med. 1999;5:938-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Kaku T, Sonoda K, Kamura T, Hirakawa T, Sakai K, Amada S, Ogawa S, Kobayashi H, Nakashima M, Watanabe T. The prognostic significance of tumor-associated antigen 22-1-1 expression in adenocarcinoma of the uterine cervix. Clin Cancer Res. 1999;5:1449-1453. [PubMed] |
| 4. | Sonoda K, Kaku T, Hirakawa T, Kobayashi H, Amada S, Sakai K, Nakashima M, Watanabe T, Nakano H. The clinical significance of tumor-associated antigen RCAS1 expression in the normal, hyperplastic, and malignant uterine endometrium. Gynecol Oncol. 2000;79:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Takahashi H, Iizuka H, Nakashima M, Wada T, Asano K, Ishida-Yamamoto A, Watanabe T. RCAS1 antigen is highly expressed in extramammary Paget's disease and in advanced stage squamous cell carcinoma of the skin. J Dermatol Sci. 2001;26:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Suzuki T, Inoue S, Kawabata W, Akahira J, Moriya T, Tsuchiya F, Ogawa S, Muramatsu M, Sasano H. EBAG9/RCAS1 in human breast carcinoma: a possible factor in endocrine-immune interactions. Br J Cancer. 2001;85:1731-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Tsuneizumi M, Nagai H, Harada H, Kazui T, Emi M. A highly polymorphic CA repeat marker at the EBAG9/RCAS1 locus on 8q23 that detected frequent multiplication in breast cancer. Ann Hum Biol. 2002;29:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Noguchi K, Enjoji M, Nakamuta M, Nakashima M, Nishi H, Choi I, Taguchi K, Kotoh K, Shimada M, Sugimachi K. Expression of a tumor-associated antigen RCAS1 in hepatocellular carcinoma. Cancer Lett. 2001;168:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Oshikiri T, Hida Y, Miyamoto M, Hashida H, Katoh K, Suzuoki M, Nakakubo Y, Hiraoka K, Shinohara T, Itoh T. RCAS1 as a tumour progression marker: an independent negative prognostic factor in gallbladder cancer. Br J Cancer. 2001;85:1922-1927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kubokawa M, Nakashima M, Yao T, Ito KI, Harada N, Nawata H, Watanabe T. Aberrant intracellular localization of RCAS1 is associated with tumor progression of gastric cancer. Int J Oncol. 2001;19:695-700. [PubMed] |
| 11. | Ohshima K, Muta K, Nakashima M, Haraoka S, Tutiya T, Suzumiya J, Kawasaki C, Watanabe T, Kikuchi M. Expression of human tumor-associated antigen RCAS1 in Reed-Sternberg cells in association with Epstein-Barr virus infection: a potential mechanism of immune evasion. Int J Cancer. 2001;93:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Izumi M, Nakanishi Y, Yoshino I, Nakashima M, Watanabe T, Hara N. Expression of tumor-associated antigen RCAS1 correlates significantly with poor prognosis in nonsmall cell lung carcinoma. Cancer. 2001;92:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Hiraoka K, Hida Y, Miyamoto M, Oshikiri T, Suzuoki M, Nakakubo Y, Shinohara T, Itoh T, Shichinohe T, Kondo S. High expression of tumor-associated antigen RCAS1 in pancreatic ductal adenocarcinoma is an unfavorable prognostic marker. Int J Cancer. 2002;99:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Matsushima T, Nakashima M, Oshima K, Abe Y, Nishimura J, Nawata H, Watanabe T, Muta K. Receptor binding cancer antigen expressed on SiSo cells, a novel regulator of apoptosis of erythroid progenitor cells. Blood. 2001;98:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Enjoji M, Nakamuta M, Noguchi K, Sugimoto R, Kotoh K, Nawata H, Nakashima M, Watanabe T. RCAS1 expression in immune-mediated liver diseases. J Clin Gastroenterol. 2002;34:286-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Japan Pancreas Society. Classification of pancreatic carcinoma. Kanahara & Co., Ltd, Tokyo, Japan. 2002;. |
| 17. | Suzuoki M, Hida Y, Miyamoto M, Oshikiri T, Hiraoka K, Nakakubo Y, Shinohara T, Itoh T, Okushiba S, Kondo S. RCAS1 expression as a prognostic factor after curative surgery for extrahepatic bile duct carcinoma. Ann Surg Oncol. 2002;9:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |