Published online Sep 7, 2005. doi: 10.3748/wjg.v11.i33.5095
Revised: October 15, 2004
Accepted: October 18, 2004
Published online: September 7, 2005
Nowadays, liver metastasis remains difficult to cure. When tumor cells escape and arrive in the liver sinusoids, they encounter the local defense mechanism specific to the liver. The sinusoidal cells have been widely described in physiologic conditions and in relation to metastasis during the past 30 years. This paper provides an “overview” of how these cells function in health and in diseases such as liver metastasis.
- Citation: Vekemans K, Braet F. Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World J Gastroenterol 2005; 11(33): 5095-5102
- URL: https://www.wjgnet.com/1007-9327/full/v11/i33/5095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i33.5095
The liver is the largest organ of the body, constituting 2-5% of the adult body weight. It receives blood supply from two major blood vessels. The hepatic artery supplies oxygenated blood, whereas the portal vein, which provides 80% of the total blood supply, supplies nutrient-rich deoxygenated blood. The liver thus acts as a guard between the digestive tract and the rest of the body[1], transforming, detoxifying, and accumulating metabolites. The liver also produces different types of plasma proteins, such as albumin, which are delivered into the blood, as well as metabolites that are constituents of the bile[2,3].
The liver is surrounded by connective tissue, designated as Glisson’s capsule. It is composed of polygonal lobules separated by connective tissue. At the periphery of the lobule, are regions that consist of bile ducts, lymphatics, nerves and branches of the hepatic artery and the portal vein. At the center of the lobule is the central vein. Hepatocytes (parenchymal cells) are the basic structural component of the liver, representing 60% of the total cell number and 80% of the total liver volume. They are arranged radially within the lobule to form cellular plates, between which the liver capillaries and the sinusoids are located.
Blood arriving through branches of the portal vein and the hepatic artery pass through the sinusoids, towards the central vein[1,4,5]. The liver sinusoids are lined with a discontinuous layer of fenestrated endothelial cells. Between the endothelial cells and the hepatocytes, is a discontinuous basal lamina and a subendothelial space named the space of Disse, through which exchange between the blood and the hepatocytes take place.
Various liver sinusoidal cells can be recognized within the sinusoids. These include, in addition to the sinusoidal endothelial cells, Kupffer cells, hepatic NK cells and stellate cells, which complement one another in performing their specialized functions.
The sinusoidal cells are the predominant non-parenchymal cells, comprising about 35% of the total cell number and about 17% of the total volume of the liver. These cells are divided into sinusoidal endothelial cells (44%), Kupffer cells (33%), stellate cells (10-25%) and hepatic NK cells (5%)[3,6-8].
The cells forming the sinusoidal wall (endothelium), the liver sinusoidal endothelial cells (LSECs), were first described at the electron microscopic level by Wisse[9]. They can be visualized by injection with fluorescent acetylated LDL (AcLDL)[15], latex beads of Ø 20 nm[16], or formaldehyde modified serum albumin (FSA)[17]. Katz et al[18], mentioned that dendritic cells are also able to take up AcLDL; however, this cannot interfere upon injection of AcLDL in the blood, as dendritic cells are to be found within the tissue and not in the blood. The liver sieve plates are a group of open pores or fenestrae lacking a diaphragm and a basal lamina underneath the endothelium. They can be visualized by perfusion fixation of the liver. These fenestrae measured 150-175 nm in transmission electron microscopy[10]. It is assumed that the transport and exchange of fluid, solutes and particles between the space of Disse and the sinusoidal blood occur through these open fenestrae[10,11].
A typical feature of endothelial cells is their high endocytic capacity[10]. This function is reflected in the presence of numerous endocytotic vesicles and in the effective uptake of a variety of substances from the blood by receptor-mediated endocytosis[10-13].
The typical LSECs phenotype, open fenestrae in sieve plates and lack of the basement membrane are maintained by paracrine and autocrine regulation[19]. CD31 has been mentioned as a de-differentiation and non-fenestration marker. In in vitro CD31 appears after 1 d monoculture; however, when co-culture with hepatocytes or stellate cells is performed, CD31 expression is prevented. This can be abolished by the addition of anti-VEGF. In accordance, VEGF stimulates the production of nitric oxide (NO)[19].
Kupffer cells are categorized as tissue macrophages and represent the largest population of their kind in the body[11]. Kupffer cells lay preferentially anchored to the periportal zone of the liver lobule[20].
Kupffer cells have irregular shapes with many cytoplasmic extensions and contain a large number of phagosomes and lysosomes that are associated with their endocytic function. Peculiarities of the ultrastructural morphology of Kupffer cells are fuzzy coat, worm-like structures, annulate lamellae and fuzzy-coated vacuoles[9,21]. Kupffer cells are scavengers that move along the sinusoids[22,23] and phagocytose foreign material present in the bloodstream; fusion of the phagosome with a lysosome leads to digestion of the ingested material. Different techniques are used to visualize these cells, such as staining for acid phosphatase activity[24] or peroxidase activity[25], and immunostaining for Fc receptors[26]. Due to their phagocytic activity, they can also be stained in vivo by injection with latex beads. Another option is staining for ED1, ED2, and ED3[27,28].
In 1976, 100 years after Kupffer cells were discovered, Wisse et al, described a new sinusoidal cell type[29]. These cells, designated as “Pit cells”, are nowadays consideredas liver-specific natural killer cells[30] with a large granular morphology[31], and can be visualized using mAb 3.2.3 against NKR-P1A[32].
Hepatic NK cells are always in contact with LSECs, and frequently in contact with Kupffer cells[10]. There is an average of 1 NK cell per 10 Kupffer cells. Hepatic NK cells can be separated into low-density and high-density cells. The two subpopulations differ morphologically, immunophenotypically and functionally from each other and from blood NK cells, particularly in the higher toxicity of high-density Kupffer cells[10,33,34]. Morphologically, NK cells can be recognized by their electron-dense, azurophilic granules and rod-cored vesicles. Rod-cored vesicles are small inclusions found exclusively in NK cells. Each vesicle contains a straight rod structure that bridges its entire diameter[10]. Hepatic NK cells have the capacity to kill incoming malignant cells[35].
Ito discovered the true function of stellate cells in 1952[36], followed by Wake[37,38]. These cells are located within the space of Disse. They contain lipid droplets rich in vitamin A, and they synthesize and secrete a variety of extra-cellular matrix proteins. The activation of stellate cells from a quiescent vitamin A storing cells to a proliferating, fibrogenic ‘myofibroblast-like’ phenotype is a main event following liver injury[39,40].
The liver is the predominant site of recurrence of the disease following initial therapeutic colon surgery, mainly due to two factors. First, viable tumor cells can escape into the portal blood stream during surgical manipulation and invade the liver[41]. Second, major surgery causes a transient postoperative weakening of the immune system[42-44]. The temporary immunosuppression induced by surgery is therefore associated with enhanced risk of metastasis. When colon cancer spreads to the liver, ablation of metastasis by surgical resection, cryotherapy or radiofrequency is the only curative treatment[45]. Moreover, the vast majority of patients are not amenable to surgical resection, due to existence of multiple metastases. In order to develop new strategies aimed at preventing metastasis, it is crucial to understand the cellular defense mechanisms against tumor cells and the tumor escape mechanisms.
When the tumor cells invade the vascular bed and metastasize into the liver, they encounter the defense mechanisms specific to the liver. Kupffer cells and hepatic NK cells are the main resident cells of immune surveillance, but sinusoidal endothelial cells also participate in this process.
Kupffer cells The most important function of Kupffer cells is defense against infections and tumor cells. Kupffer cells act as antigen presenting cells and as effector cells that act directly by phagocytosis, or indirectly by activation of other cells, e.g. NK cells[46,47]. Though Kupffer cells are constantly acting as scavengers, they can be activated through different pathways. Firstly, soluble mediators can trigger their activation. IFN-γ is the prototypical macrophage activating factor. It is central to the development of Th1-dominated immune responses, and it affects not only macrophages in an autocrine fashion, but other immune cells as well.
One of the key events during innate immune reactions is the production of IL-12, mainly by macrophages[48]. IL-12 induces NK cells to rapidly secrete IFN-γ, which then in turn activates macrophages early in the immune response. It also induces IFN-γ production by T cells. IL-18, which is produced by Kupffer cells and other cell types, is also involved in enhancing IFN-γ production by T cells[49,50]. Macrophages also secrete IFN-γ upon stimulation with IL-12 and IL-18 together. It is known that IFN-γ is a possible reducer of metastasis of colon cancer in the liver[51].
Macrophages can also be activated by direct interaction with micro-organisms or bacterial products such as lipopoly-saccharide, glucan, muramyl dipeptide and lipid A[52]. A pivotal role for IFN-γ in the clearance of various intracellular pathogens has been amply demonstrated[53,54]. It has been described that macrophages release the cytotoxic radical, NO[55,56]. In vitro studies suggested that NO induces mitochondrial dysfunction in tumor cells followed by membrane barrier dysfunction in the liver sinusoid[57,58]. Another important cytotoxic factor released by activated macrophages is tumor necrosis factor alpha (TNF-α), which is produced in both soluble and membrane-bound forms. After binding to its receptor, apoptosis can be induced in the target cell[59].
Cytotoxicity of macrophages can be classified into antibody-dependent and antibody-independent cell-mediated cytotoxicity. Both pathways are contact dependent and induce tumor cell death after a number of hours. Antibody-dependent cell-mediated cytotoxicity is based on the recognition of an antibody-coated target by Fc receptors on the effector cells[60-62]. Upon cross-linking of the Fc receptor, secretion of cytotoxic mediators occurs. Secretion of reactive oxygen species, IL-1 and TNF-α are probably involved[63]. Antibody-independent cell-mediated cytotoxicity involves binding to the macrophage followed by translocation of the lysosomal organelles to the target[47]. Moreover, cytotoxicity towards tumor targets involves cytolysis and phagocytosis[64].
In certain pathophysiologic conditions, apoptosis is chaotic and non-selective, may be massive and occurs persistently over an extended period of time[65]. A large number of apoptotic bodies produced are phagocytosed by Kupffer cells. It has recently been reported that engulfment of apoptotic bodies results in the generation of death ligands, such as FasL and TNF-α on the membrane of the Kupffer cells, but engulfment of latex beads does not produce a similar response[59]. This means that uptake of apoptotic bodies induces an additional immunologic response involving liver inflammation and fibrosis[59,66].
Hepatic NK cells Cytotoxic lymphocytes (CTL) and NK cells induce target cell death by means of granules and by death receptor-mediated pathways. Apoptosis refers to orchestrated cell death that is indispensable for maintenance of homeostasis. Characteristics of apoptosis are chromatin condensation, nuclear fragmentation, membrane blebbing, cell shrinkage, protein degradation and internucleosomal DNA fragmentation. Most of the morphological changes are caused by a set of cysteine proteases that are activated specifically in apoptotic cells. These death proteases are homologous to each other and are members of a large protein family known as caspases[67]. Over a dozen caspases have been identified in humans, and it has been suggested that about two-thirds of them function in apoptosis[68,69]. Different pathways can induce apoptosis, of which the Fas/FasL pathway is the best known. This pathway is frequently used by cytotoxic T cells and other immunocompetent cells. Stimulation of the Fas receptor (CD95) with Fas ligand recruits the proform of the initiator caspase 8, by interaction with the adapter molecule Fas-associated death domain through death domains and death effector domains. This leads to the formation of a complex called the death inducing signaling complex. Subsequently, procaspase 8 is cleaved autocatalytically to yield the active initiator caspase 8. The consequences of the activation of caspase 8 depend on the cell type. In type 1 cells, the other members of the caspase family (such as caspase 3) are activated directly[70]. In type 2 cells, caspase 8 activation results in the cleavage of the pro-apoptotic Bcl-2 family member Bid. Subsequently Bid and Bax are translocated to the mitochondria to cause release of cytochrome c. This results in the activation of caspase 9 through interaction with the adapter molecule apoptotic protease-activating factor. Caspase 9 activates caspase 3, which can then perform its function[71,72].
The granules of CTL contain various proteins, some of which are known to be involved in target cell death, such as perforin and granzyme. Various isoforms of the granzymes have been described. After granzyme B enters the target cell, it can directly cleave procaspase 3, Bid and inhibitor of caspase-activated DNase, thereby inducing apoptosis[71,73-75]. Death induced by granzyme A appears to be independent of the pathways used by granzyme B[75,76]. Granzyme A does not activate caspases in target cells, nor does it induce cleavage of other granzyme B substrates. The functions of the other granzyme isoforms remain unclear[76]. Perforin forms transmembrane pores and is an essential enabler of granzyme-mediated apoptosis[77].
Liver sinusoidal endothelial cells LSECs, like Kupffer cells, express an Fc receptor that may be involved in immunological defense. FcR-mediated uptake of IgG-immune complex has been shown to enhance presentation of antigens by MHC class II[78]. This process might take place in a compartment reminiscent of the multi-vesicular compartments observed in the LSECs. It is therefore conceivable that these organelles are involved in processing and presentation of antigenic peptides. In contrast to Kupffer cells, however, the multi-vesicular compartments observed in LSECs are not rich in MHC class II, which might indicate that LSECs are not involved in antigen presentation[79-81]. Nevertheless, Knolle et al.[82,83], demonstrated that both Kupffer cells and LSEC have MHC class II, and that both of these cells are involved in antigen presentation. However, LSECs failed to induce differentiation towards inflammatory Th1[82]. Furthermore, antigen presentation of soluble blood-borne antigens leads primarily to tolerance, instead of immunosurveillance. LSECs are known to express CD80 and CD86, molecules that are present on professional antigen-presenting cells, such as dendritic cells[14]. In addition, LSECs play a key role in receptor-mediated uptake, in degradation of macromolecules from the sinusoidal blood[84-86] and in the clearance of circulating apoptotic bodies[83,84]. Katz et al[18], reported about some conflicting data, low or absent MHC class II, CD86 and CD11c. However, they mentioned a high capacity for AG uptake in vivo and in vitro. According to Knolle et al[14,83], LSECs were unable to stimulate allogeneic T cells.
LSECs are known to secrete NO and can induce apoptosis in different types of cancer cells, such as lymphoma and colorectal carcinoma[87,88]. NO has also been reported to function as a cytolytic factor in macrophage-mediated cancer cell cytotoxicity[89].
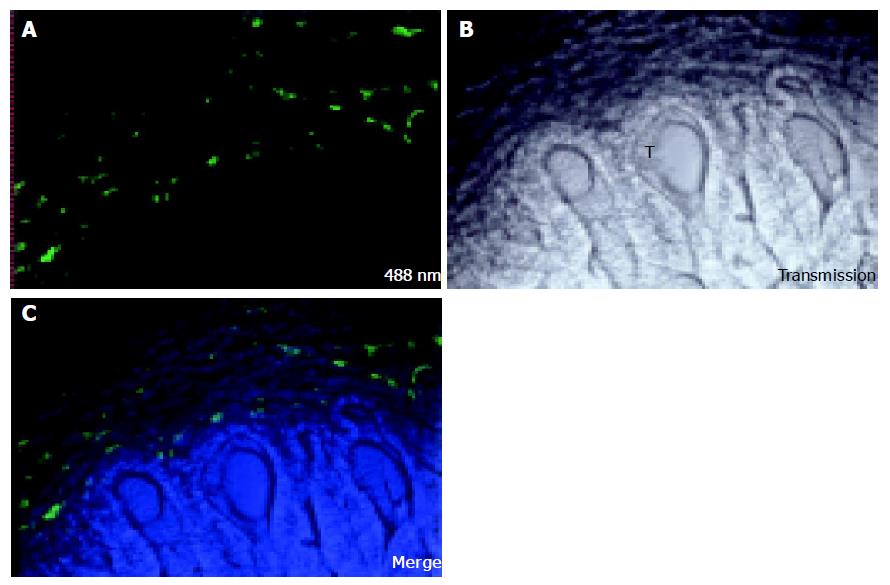
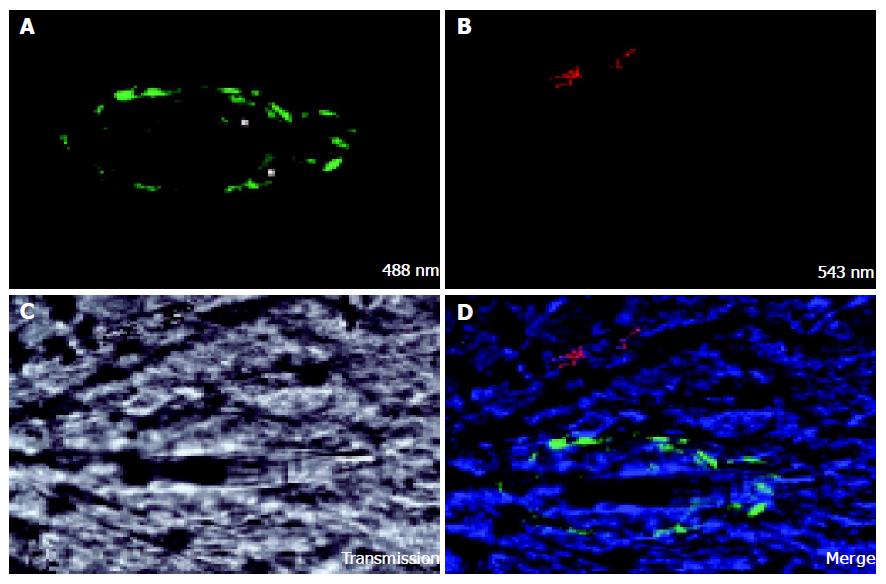
Seventeen days after injection of colon carcinoma cells CC531s into the mesenteric vein, metastases are observed as nodules of 1-3 mm diameter on the surface of the liver lobes. When the liver sinusoidal cells are stained in vivo with FSA, no staining can be detected in the center of the metastasis nodule (Figure 1). On the border of the tumor some positive staining can be seen, but of lower intensity than in normal tissue. It is known that nodules of 1-2 mm diameter have no internal vascularization, and that the cells receive nutrients and oxygen simply by diffusion (prevascular phase)[90]. Vessel formation, however, was observed with confocal microscopy in a cell cluster of 12 CC531s cells 24 h after injection of CC531s cells (Figure 2) and it is known that colon carcinoma cells produce angiogenic factors such as VEGF[91]. However, even though angiogenic factors permit formation of vessels in micro-metastases as shown in Figure 2, these vessels are incapable of extending to provide a blood supply within macro-metastasis nodules as shown in Figure 1.
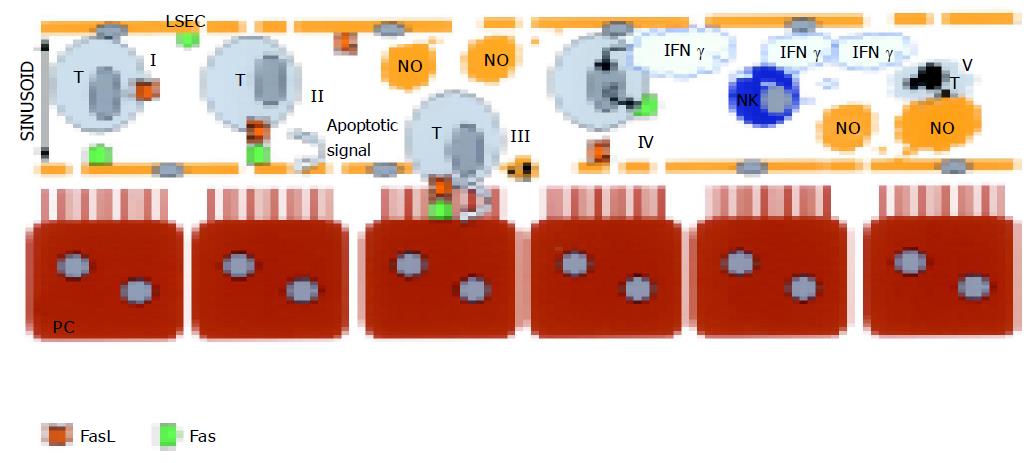
In conclusion, with our published studies, we add these results that most of the CC531s cells trapped in the sinusoids get phagocytosed by the Kupffer cells and only 6% of the cells are living in the sinusoid or in the space of Disse[92]. Hepatic NK cells help in the removal of the CC531s. In NK cells, depleted rates up to 33% of the CC531s are free of interactions with Kupffer cells[92]. In order to understand the pathways involved, in vitro studies were conducted and the following conclusions were made. CC531s, colon carcinoma cells, are able to kill LSECs by usage of Fas/FasL pathway[94] (Figure 3). In vivo, destruction of the sinusoidal lining can be observed and is also confirmed by others[93-95]. However, when CC531s are pre-treated with IFN-γ, the outcome of apoptosis in cocultures is inversed. Then, LSECs are able to induce apoptosis in pre-treated CC531s by NO pathway[96,97] (Figure 3). Generally we state that Kupffer cells, NK cells, and LSECs orchestrate together in the removal of CC531s.
We want to thank Mrs. C. Seynaeve, M. Baekeland, D. Blijweert, and Mrs. Chris Derom for excellent technical assistance. Also thanks to Dr. B. Smedsrød for giving the FSA. The members of the “Australian Key Center for Microscopy and Microanalysis” of The University of Sydney are gratefully acknowledged for excellent administrative, technical, and practical support.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Junqueira LC, Carneiro J. Basic Histology 10th edition Lange international edition. 332-343. |
| 2. | Arias IM, jakoby WB, Popper H, Scachter D, Schafritz DA. Section 4 the organ: The hepatic microvascular system. The liver: Biology and Pathobiology, 3rd Ed. Lippincott Williams & Wilkins 1997; Available from: http: //liver.med.tufts.edu/. |
| 4. | Lautt WW, Greenway CV. Conceptual review of the hepatic vascular bed. Hepatology. 1987;7:952-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 233] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Muto M. A scanning electron microscopic study on endothelial cells and Kupffer cells in rat liver sinusoids. Arch Histol Jpn. 1975;37:369-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Knook DL, Sleyster EC. Isolated parenchymal, Kupffer and endothelial rat liver cells characterized by their lysosomal enzyme content. Biochem Biophys Res Commun. 1980;96:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 638] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Weibel ER, Stäubli W, Gnägi HR, Hess FA. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969;42:68-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 794] [Cited by in RCA: 832] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 427] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Braet F, Luo D, Spector I, Vermijlen D, Wisse E. Endothelial and pit cells. The liver: Biology and pathobiology, 4 ed. Philadelphia: Lippincott Williams Wilkins 2001; 437-453. |
| 11. | Smedsrød B, De Bleser PJ, Braet F, Lovisetti P, Vanderkerken K, Wisse E, Geerts A. Cell biology of liver endothelial and Kupffer cells. Gut. 1994;35:1509-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Eskild W, Smedsrod B, Berg T. Receptor mediated endocytosis of formaldehyde treated albumin, yeast invertase and chondroitin sulfate in suspensions of rat liver endothelial cells. Int J Biochem. 1986;18:647-651. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Eskild W, Kindberg GM, Smedsrod B, Blomhoff R, Norum KR, Berg T. Intracellular transport of formaldehyde-treated serum albumin in liver endothelial cells after uptake via scavenger receptors. Biochem J. 1989;258:511-520. [PubMed] |
| 14. | Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Pitas RE, Boyles J, Mahley RW, Bissell DM. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol. 1985;100:103-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 183] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Shiratori Y, Tananka M, Kawase T, Shiina S, Komatsu Y, Omata M. Quantification of sinusoidal cell function in vivo. Semin Liver Dis. 1993;13:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Yoshioka T, Yamamoto K, Kobashi H, Tomita M, Tsuji T. Receptor-mediated endocytosis of chemically modified albumins by sinusoidal endothelial cells and Kupffer cells in rat and human liver. Liver. 1994;14:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757-G763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 268] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Wisse E. An ultrastructural characterization of the endothelial cell in the rat liver sinusoid under normal and various experimental conditions, as a contribution to the distinction between endothelial and Kupffer cells. J Ultrastruct Res. 1972;38:528-562. [RCA] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 190] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Kan Z, Ivancev K, Lunderquist A, McCuskey PA, McCuskey RS, Wallace S. In vivo microscopy of hepatic metastases: dynamic observation of tumor cell invasion and interaction with Kupffer cells. Hepatology. 1995;21:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | MacPhee PJ, Schmidt EE, Groom AC. Evidence for Kupffer cell migration along liver sinusoids, from high-resolution in vivo microscopy. Am J Physiol. 1992;263:G17-G23. [PubMed] |
| 24. | Matsubara S. Glucose-6-phosphate dehydrogenase and mouse Kupffer cell activation: an ultrastructural dual staining enzyme-cytochemical study. Histochem Cell Biol. 2002;118:345-350. [PubMed] |
| 25. | Albegger KW. Sturcture and function of the mononuclear phagocytic system (MPS) in chronic rhinosinusitis. A light and electron microscopic investigation (author's transl). Arch Otorhinolaryngol. 1976;214:27-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Wood GW, Gollahon KA. T-lymphocytes and macrophages in primary murine fibrosarcomas at different stages in their progression. Cancer Res. 1978;38:1857-1865. [PubMed] |
| 27. | Dijkstra CD, Döpp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589-599. [PubMed] |
| 28. | Ukai K, Terashima K, Imai Y, Shinzawa H, Okuyama Y, Takahashi T, Ishikawa M. Proliferation kinetics of rat Kupffer cells after partial hepatectomy. Immunohistochemical and ultrastructural analysis. Acta Pathol Jpn. 1990;40:623-634. [PubMed] |
| 29. | Wisse E, van't Noordende JM, van der Meulen J, Daems WT. The pit cell: description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173:423-435. [RCA] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Bouwens L, Wisse E. Immuno-electron microscopic characterization of large granular lymphocytes (natural killer cells) from rat liver. Eur J Immunol. 1987;17:1423-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Nakatani K, Kaneda K, Seki S, Nakajima Y. Pit cells as liver-associated natural killer cells: morphology and function. Med Electron Microsc. 2004;37:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | van den Brink MR, Hunt LE, Hiserodt JC. In vivo treatment with monoclonal antibody 3.2.3 selectively eliminates natural killer cells in rats. J Exp Med. 1990;171:197-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Vanderkerken K, Bouwens L, Wisse E. Characterization of a phenotypically and functionally distinct subset of large granular lymphocytes (pit cells) in rat liver sinusoids. Hepatology. 1990;12:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Luo D, Vanderkerken K, Bouwens L, Kuppen PJ, Baekeland M, Seynaeve C, Wisse E. The role of adhesion molecules in the recruitment of hepatic natural killer cells (pit cells) in rat liver. Hepatology. 1996;24:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Vermijlen D, Luo D, Robaye B, Seynaeve C, Baekeland M, Wisse E. Pit cells (Hepatic natural killer cells) of the rat induce apoptosis in colon carcinoma cells by the perforin/granzyme pathway. Hepatology. 1999;29:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Ito T, Nemoto M. [Kupfer's cells and fat storing cells in the capillary wall of human liver]. Okajimas Folia Anat Jpn. 1952;24:243-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 88] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Wake K. "Sternzellen" in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat. 1971;132:429-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 256] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303-353. [RCA] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 368] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Li D, Friedman SC: hepatic stellate cells: morphology, function , and regulation. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter DA, Shafritz DA, eds. The liver: biology and pathobiology, 4 ed. Philadelphia: Lippincott Williams Wilkins 2001; 455-468. |
| 41. | Roberts S, Long L, Jonasson O, McGrath R, McGrew E, Cole WH. The isolation of cancer cells from the blood stream during uterine curettage. Surg Gynecol Obstet. 1960;111:3-11. [PubMed] |
| 42. | Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Faist E, Mewes A, Strasser T, Walz A, Alkan S, Baker C, Ertel W, Heberer G. Alteration of monocyte function following major injury. Arch Surg. 1988;123:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 162] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol. 2002;29:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, Proud G, Taylor RM. The influence of surgical operations on components of the human immune system. Br J Surg. 1985;72:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 258] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Unanue ER, Allen PM. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987;236:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 716] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 47. | Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1120] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 48. | Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1757] [Cited by in RCA: 1792] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 49. | Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2208] [Cited by in RCA: 2216] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 50. | Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 457] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 51. | Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 558] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 52. | Rushfeldt C, Sveinbjørnsson B, Seljelid R, Smedsrød B. Early events of hepatic metastasis formation in mice: role of Kupffer and NK-cells in natural and interferon-gamma-stimulated defense. J Surg Res. 1999;82:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Schultz RM, Chirigos MA. Macrophage activation for nonspecific tumor cytotoxicity. Adv Pharmacol Chemother. 1980;17:157-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103-2108. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 55. | Gaillard T, Mülsch A, Busse R, Klein H, Decker K. Regulation of nitric oxide production by stimulated rat Kupffer cells. Pathobiology. 1991;59:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Yonei Y, Kurose I, Fukumura D, Saito H, Miura S, Tsukada N, Oda M, Tsuchiya M. Evidence of direct interaction between Kupffer cells and colon cancer cells: an ultrastructural study of the co-culture. Liver. 1994;14:37-44. [PubMed] |
| 57. | Kurose I, Miura S, Higuchi H, Watanabe N, Kamegaya Y, Takaishi M, Tomita K, Fukumura D, Kato S, Ishii H. Increased nitric oxide synthase activity as a cause of mitochondrial dysfunction in rat hepatocytes: roles for tumor necrosis factor alpha. Hepatology. 1996;24:1185-1192. [PubMed] |
| 58. | Fukumura D, Yonei Y, Kurose I, Saito H, Ohishi T, Higuchi H, Miura S, Kato S, Kimura H, Ebinuma H. Role in nitric oxide in Kupffer cell-mediated hepatoma cell cytotoxicity in vitro and ex vivo. Hepatology. 1996;24:141-149. [PubMed] |
| 59. | Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 60. | Fanger MW, Shen L, Graziano RF, Guyre PM. Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today. 1989;10:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 263] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | van de Winkel JG, Anderson CL. Biology of human immunoglobulin G Fc receptors. J Leukoc Biol. 1991;49:511-524. [PubMed] |
| 62. | Løvdal T, Andersen E, Brech A, Berg T. Fc receptor mediated endocytosis of small soluble immunoglobulin G immune complexes in Kupffer and endothelial cells from rat liver. J Cell Sci. 2000;113:3255-3266. [PubMed] |
| 63. | Loegering DJ, Lennartz MR. Differential effect of Fc gamma receptor ligation on LPS-stimulated TNF-alpha secretion by hepatic, splenic, and peritoneal macrophages. Inflammation. 2002;26:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 64. | Gardner CR, Wasserman AJ, Laskin DL. Differential sensitivity of tumor targets to liver macrophage-mediated cytotoxicity. Cancer Res. 1987;47:6686-6691. [PubMed] |
| 65. | Patel T, Roberts LR, Jones BA, Gores GJ. Dysregulation of apoptosis as a mechanism of liver disease: an overview. Semin Liver Dis. 1998;18:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Jaeschke H. Inflammation in response to hepatocellular apoptosis. Hepatology. 2002;35:964-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1686] [Cited by in RCA: 1653] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 68. | Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 2003] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 69. | Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5088] [Article Influence: 188.4] [Reference Citation Analysis (0)] |
| 70. | Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2314] [Cited by in RCA: 2232] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 71. | Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2:401-409. [PubMed] |
| 72. | Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1161] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 73. | Yang X, Stennicke HR, Wang B, Green DR, Jänicke RU, Srinivasan A, Seth P, Salvesen GS, Froelich CJ. Granzyme B mimics apical caspases. Description of a unified pathway for trans-activation of executioner caspase-3 and -7. J Biol Chem. 1998;273:34278-34283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Thomas DA, Du C, Xu M, Wang X, Ley TJ. DFF45/ICAD can be directly processed by granzyme B during the induction of apoptosis. Immunity. 2000;12:621-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 761] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 76. | Beresford PJ, Xia Z, Greenberg AH, Lieberman J. Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity. 1999;10:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 877] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 78. | Manca F, Fenoglio D, Li Pira G, Kunkl A, Celada F. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. J Exp Med. 1991;173:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Pulford K, Souhami RL. The surface properties and antigen-presenting function of hepatic non-parenchymal cells. Clin Exp Immunol. 1981;46:581-588. [PubMed] |
| 80. | Smedsrod B, Pertoft H, Eggertsen G, Sundstrom C. Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res. 1985;241:639-649. [RCA] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Løvdal T, Andersen E, Brech A, Berg T. Fc receptor mediated endocytosis of small soluble immunoglobulin G immune complexes in Kupffer and endothelial cells from rat liver. J Cell Sci. 2000;113:3255-3266. [PubMed] |
| 82. | Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S, Lohse AW, Gerken G. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J Immunol. 1999;162:1401-1407. [PubMed] |
| 83. | Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, Gerken G, Lohse AW. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Hansen B, Melkko J, Smedsrød B. Serum is a rich source of ligands for the scavenger receptor of hepatic sinusoidal endothelial cells. Mol Cell Biochem. 2002;229:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 85. | Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, Vidal-Vanaclocha F. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108:967-973. [PubMed] |
| 86. | Bogers WM, Stad RK, Janssen DJ, van Rooijen N, van Es LA, Daha MR. Kupffer cell depletion in vivo results in preferential elimination of IgG aggregates and immune complexes via specific Fc receptors on rat liver endothelial cells. Clin Exp Immunol. 1991;86:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Edmiston KH, Shoji Y, Mizoi T, Ford R, Nachman A, Jessup JM. Role of nitric oxide and superoxide anion in elimination of low metastatic human colorectal carcinomas by unstimulated hepatic sinusoidal endothelial cells. Cancer Res. 1998;58:1524-1531. [PubMed] |
| 88. | Rocha M, Kruger A, Van Rooijen N, Schirrmacher V, Umansky V. Liver endothelial cells participate in T-cell-dependent host resistance to lymphoma metastasis by production of nitric oxide in vivo. Int J Cancer. 1995;63:405-411. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Kurose I, Miura S, Fukumura D, Yonei Y, Saito H, Tada S, Suematsu M, Tsuchiya M. Nitric oxide mediates Kupffer cell-induced reduction of mitochondrial energization in hepatoma cells: a comparison with oxidative burst. Cancer Res. 1993;53:2676-2682. [PubMed] |
| 90. | Strohmeyer D. Pathophysiology of tumor angiogenesis and its relevance in renal cell cancer. Anticancer Res. 1999;19:1557-1561. [PubMed] |
| 91. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6489] [Article Influence: 259.6] [Reference Citation Analysis (0)] |
| 92. | Timmers M, Vekemans K, Vermijlen D, Asosingh K, Kuppen P, Bouwens L, Wisse E, Braet F. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer. 2004;112:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Vekemans K, Timmers M, Vermijlen D, De Zanger R, Wisse E, Braet F. CC531s colon carcinoma cells induce apoptosis in rat hepatic endothelial cells by the Fas/FasL-mediated pathway. Liver Int. 2003;23:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Vekemans K, Braet F, Wisse E. CC531S-induced damage of the rat liver sinusoidal endothelial lining is mediated by the Fas/FasL pathway. Hepatology. 2003;38:1314; author reply 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 95. | Mook OR, Van Marle J, Vreeling-Sindelárová H, Jonges R, Frederiks WM, Van Noorden CJ. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology. 2003;38:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 96. | Vekemans K, Braet F, Muyllaert D, Wisse E. Nitric oxide from rat liver sinusoidal endothelial cells induces apoptosis in IFN gamma-sensitized CC531s colon carcinoma cells. J Hepatol. 2004;41:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 97. | Vekemans K, Braet F, Wisse E. DiO-labeled CC531s colon carcinoma cells traverse the hepatic sinusoidal endothelium via the Fas/FasL pathway. J Gastrointest Surg. 2004;8:371-372; author reply 372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |