INTRODUCTION
Cholangiocarcinoma (CCC) is the second most common primary liver cancer after hepatocellular carcinoma[1]. Its prognosis is poor. The 5-year survival after surgical treatment with curative intention is 10-30%[2], but at the time of diagnosis less than one-third of tumors are resectable[3,4]. Single agent or combination chemotherapy and conventional radiation therapy are so far not effective, neither as primary treatment nor as adjuvant treatment after resection[1,5]. The majority of patients survive less than 12 mo after diagnosis[5,6]. Thus, there is a strong need for new therapeutic strategies. Here we report a 45-year-old patient with inoperable hilar CCC, who was treated using an inter-disciplinary concept including stereotactic radiotherapy. This highly precise technique also known as extracranial stereotactic radiotherapy[7], was first developed for the treatment of brain tumors. Using a body frame, it is applicable to the whole body. By this technique, an adequate radiation dose can be delivered to the tumor, while sparing normal structures in the liver hilus and the upper abdomen that are highly radiosensitive. A total dose of 48 Gy (3×4 Gy/wk) could be given and therapy is well tolerated. Although the tumor is locally advanced at the time of diagnosis and prognosis was extremely poor, an unusual long survival of more than 4 years with a good quality of life could be achieved. Stereotactic radiotherapy seems to be a promising choice of treatment for hilar CCC.
CASE REPORT
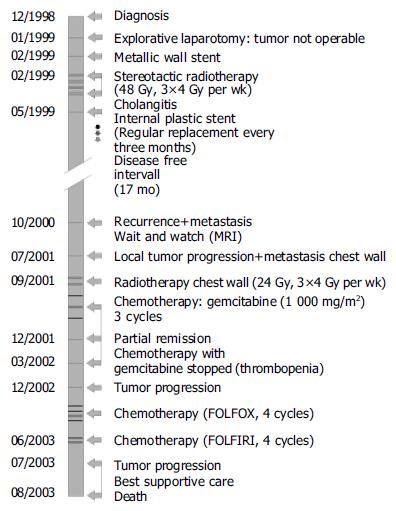
In December 1998, a 45-year-old male patient was admitted to our hospital. The only pathologic finding at clinical examination was a generalized, painless jaundice. The patient reported itching, but no abdominal pain, no fever, and no weight loss. Laboratory tests showed elevated levels of alkaline phosphatase (591 U/L; normal 35-104 U/L), γ-glutamyltransferase (75 U/L; normal <39 U/L) and bilirubin (22.8 mg/dL; normal <1.2 mg/dL). C-reactive protein increased slightly (15 mg/L; normal <5 mg/L). The tumor antigen CA 19-9 was elevated (405 U/L; normal <37 U/L). All other routine biochemical tests were normal. Abdominal ultrasound examination showed dilation of intra-hepatic and extra-hepatic bile ducts. Computer tomography (CT) and magnetic resonance imaging (MRI) revealed a 6 cm×7 cm tumor in hepatic duct bifurcation and cholestasis predominantly in the atrophic left lobe of the liver. Several enlarged lymph nodes were detected in the hepato-duodenal ligament and one retro-pancreatic lymph node in contact to the inferior vena cava. Endoscopic retrograde cholangiopancreaticography (ERCP) showed a stenosis of the hepatic duct bifurcation. A biopsy was taken and using percutaneous transhepatic cholangial drainage (PTCD), an external-internal Yamakawa drainage was placed. Histological examination revealed a highly differentiated CCC. Chest X-ray showed no evidence for metastases. Explorative laparotomy showed a non-resectable tumor. The tumor was classified as American Joint Committee on Cancer stage III according to the staging system for CCC[8] and as type IV according to modified Bismuth-Corlette classification for hilar CCC[9,10]. The biliary drain was internalized post-operatively by implanting a metallic Memotherm stent (60 mm/10 mm, Fa. Bard-Angiomed, Karlsruhe, Germany). Subsequently a stereotactic radiotherapy with 48 Gy was performed for over 4 wk (3×4 Gy/wk) using the technique of Lax et al[7,11], with slight modifications. After careful fixation of the patient in a body frame, treatment planning was performed in series of 45 axial CT slices with a distance and thickness of 5 mm. The planning target volume[12,13], including the clinical target volume (tumor and involved structures/lymphatic nodes) at a distance between 3 and 5 mm to compensate for organ motion and repositioning errors, was 370 cm³. To check the repositioning accuracy in the body frame, a second CT series was performed few days after the first. The maximum positioning deviation in the region of the target volume was less than 3 mm. An optimal dose distribution could be achieved with seven coplanar conformal photon beams from a 6 MV linear accelerator (Figure 1). Using this technique, the mean dose values to liver, right kidney, spleen, and duodenum could be kept at 20.2, 3.2, 8.7, and 22.2 Gy, respectively. The patient developed cholangitis 2 mo after radiotherapy. An ERCP showed a stenosis close to the papilla Vateri. A biopsy was not conclusive and could not determine whether the stenosis was caused by tumor or radiation. The patient was treated with antibiotics. An internal plastic stent (10F/7 cm, Endo-Flex GmbH, Voerde, Germany) was placed to relieve the distal biliary obstruction. Jaundice and associated symptoms resolved. The patient was followed up at 3-mo intervals. Because transhepatic biliary stents have a 1-year patency rate of only 50%[14,15], the stent was replaced endoscopically every 3 mo between May 1999 and July 2003. MRI, in November 1999, showed no contrast medium enhancement in the tumor region and stable size of the lymph nodes. MRI, one year later (October 2000), showed constant size of the tumor, but a slightly contrast medium enhancement and enlargement of one retro-pancreatic lymph node consistent with a slowly growing residual tumor. In addition, a metastasis was detected in the right lobe of the liver. The patient refused chemotherapy. MRI, in April 2001, showed stable disease. In July 2001, the patient reported a weight loss of 18 kg in 2 mo and pain in the right chest. MRI revealed tumor progression and a metastasis in the right chest wall. Therefore, a combined palliative radiochemotherapy was initiated in September 2001. The chest wall metastasis was irradiated with a photon beam of a 6 MV linear accelerator with a total dose of 24 Gy (3×4 Gy/wk). Chemotherapy with gemcitabine 1 000 mg/m2 was initiated. MRI, after three cycles of chemotherapy in December 2001, showed partial remission with regression of all lesions. Therefore, chemotherapy was continued. Because of thrombocytopenia and anemia, only two additional cycles of gemcitabine at a reduced dose could be given and chemotherapy had to be stopped in March 2002. After tumor progression, the patient received four cycles of 5-fluorouracil/leucovorin/oxaliplatin FOLFOX[7] and two cycles of 5-fluorouracil/leucovorin/irinotecan FOLFIRI. In July 2003, MRI showed significant progression of the tumor. Though the tumor had such a final progression, the patient had a good quality of life for more than 4 years. Four years and six months after diagnosis he died of liver failure. The treatment course of the patient is shown in Figure 2.
Figure 1 CT slice of radiotherapy treatment plan in the drainage area (planning system: TMS Helax).
1-7: Coplanar beams. Isodoses: green: 100% = 48.0 Gy; light blue 1: 90% = 43.2 Gy; light blue 2: 80% = 38.4 Gy; light blue 3: 70% = 33.6 Gy; dark blue: 50% = 24.0 Gy. A: PTV (planning target volume): outer red line includes a safety rim. B: Body frame (low density materials to avoid shielding) with positioning marks. C: Vacuum cushion for reproducible fixation of the patient (low density materials to avoid shielding: not visible in CT scan). D: Drainage. E: Radio-opaque fiducials for the coordinate read out in longitudinal direction.
Figure 2 Patient’s treatment course from 12/1998 to 08/2003.
DISCUSSION
Patients with CCC have an extremely poor prognosis. Because of the high recurrence rate of CCC, there is no indication for liver transplantation[14,16,17]. Thus, surgical resection remains the only potentially curative treatment with a 5-year survival rate of 0-22% (mean 14%) for hilar CCC and 0-39% (mean 25%) for distal CCC[18]. At the time of diagnosis, however, resection is possible in less than a third of the patients[3]. If the tumor is unresectable, therapeutic interventions are directed toward the relief of biliary obstruction and its associated symptoms. Palliative options include ERC, PTCD, or a bilio-digestive anastomosis. Endoscopic biliary drainage with self-expandable metal stents has become the favored palliative drainage procedure and can be successfully performed on most patients with hilar CCC[5]. Metallic wall stents are preferred over plastic stents because of their longer patency[19-21]. In a small number of patients with unresectable CCC and failed endoscopic stents, photodynamic therapy (PDT) has been used. This technique involves the intravenous application of a photosensitizer followed by intra-luminal cholangioscopic photoactivation and generation of oxygen free radicals that harm cancer cells preferentially. Therapy decreases bilirubin levels and leads to a slightly better survival rate[22,23]. It was reported that bilary stenting with and without PDT shows a survival benefit for patients receiving PDT[24]. However, a puzzling aspect of the study is the failure to relieve bile duct obstruction with stenting alone. Therefore the observed survival benefit is probably caused by relief of cholestasis rather than by a reduction of tumor and it remains unclear, if PDT prolongs survival in patients responding to conventional bilary stenting procedures. Although this new technique appears promising, further studies are needed. Chemotherapy has not been shown to have a significant impact on survival[25]. The majority of reports use 5-FU alone or in combination with leucovorin, methotrexate, cisplatin, mitomycin C, or interferon alpha[5]. In some phase II studies, a response rate of about 20% is reported for gemcitabine[26,27]. The majority of reports concerning chemotherapy in patients with CCC are retrospective and include only few patients. In intra-hepatic CCC, transarterial chemoperfusion in combination with temporary embolization of the feeding artery can be performed. But randomized controlled trials demonstrating an impact on survival are lacking. Few studies have reported benefits of primary or adjuvant standard radiation therapy[28,29]. There are no randomized controlled trials demonstrating a therapeutic effect of brachytherapy or external beam radiotherapy[5]. The main problem of radiation therapy of the upper abdomen is to deliver an adequate dose to the tumor without serious side effects. Using a conforming, stereotactic radiation technique, a steep dose gradient can be obtained[30]. This means that a high radiation dose reaches the tumor while the normal tissue around the target volume is irradiated with comparatively low doses. Such a highly precise dose distribution is a need in radiotherapy of the liver hilus. Apart from the dose distribution the repositioning accuracy of the target volume is central to a successful radiation therapy. The high repositioning accuracy by a body frame[7,11] can explain the differences between our patient and published series with respect to side effects and tumor regression. Thus, stereotactic radiotherapy using a body frame is a valuable option for non-invasive treatment of inoperable tumors in critical regions as the liver hilus. Further technical developments as image guided radiotherapy or breath-triggered radiotherapy should further increase the repositioning accuracy and may make this technique available for routine use.
In conclusion, considering the generally poor prognosis of patients with Klatskin tumor, the long-term survival of our patient treated by stereotactic radiotherapy should encourage a systematic evaluation of this therapeutic strategy by prospective and randomized protocols.