Published online Aug 21, 2005. doi: 10.3748/wjg.v11.i31.4883
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: August 21, 2005
AIM: α-Lipoic acid (ALA) has been used as an antioxidant. The aim of this study was to investigate the effect of α-lipoic acid on cholecystokinin (CCK)-octapeptide induced acute pancreatitis in rats.
METHODS: ALA at 1 mg/kg was intra-peritoneally injected, followed by 75 μg/kg CCK-octapeptide injected thrice subcutaneously after 1, 3, and 5 h. This whole procedure was repeated for 5 d. We checked the pancreatic weight/body weight ratio, the secretion of pro-inflammatory cytokines and the levels of lipase, amylase of serum. Repeated CCK octapeptide treatment resulted in typical laboratory and morphological changes of experimentally induced pancreatitis.
RESULTS: ALA significantly decreased the pancreatic weight/body weight ratio and serum amylase and lipase in CCK octapeptide-induced acute pancreatitis. However, the secretion of IL-1β, IL-6, and TNF-α were comparable in CCK octapeptide-induced acute pancreatitis.
CONCLUSION: ALA may have a protective effect against CCK octapeptide-induced acute pancreatitis.
- Citation: Park SJ, Seo SW, Choi OS, Park CS. α-Lipoic acid protects against cholecystokinin-induced acute pancreatitis in rats. World J Gastroenterol 2005; 11(31): 4883-4885
- URL: https://www.wjgnet.com/1007-9327/full/v11/i31/4883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i31.4883
Acute pancreatitis (AP) is a clinical entity that is believed to have intracellular activation of digestive enzymes and autodigestion of the pancreas as its central patho-physiologic cause. This non-infectious destruction of pancreatic parenchyma quickly induces an inflammatory reaction at the site of injury. AP usually occurs as a result of alcohol abuse. Histologically, acute pancreatitis is characterized by interstitial edema, vacuolization, inflammation and acinar cell necrosis[1,2]. The diagnosis of acute pancreatitis is usually based on pancreatic edema index (pancreatic weight/ body weight), pancreatic serum enzymes (e.g. pancreatic amylase, lipase, immunoreactive trypsin or elastase) at animal models[3,4].
Cytokines are important immunoregulatory mediators. Their contribution to the pathogenesis of acute and chronic gastroenterological disorders is obvious. Increased expression of interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor (TNF)-α can be detected in AP. These cytokines are involved in the pathogenesis of pancreatitis-associated multiple organ dysfunction.
Among the neurohormonal regulators, Cholecystokinin (CCK) is well known gastrointestinal hormone and neural agonist for inducing the release of pancreatic digestive enzymes. At supra-maximal doses (dose greater than those that cause maximal secretion of digestion enzyme by the pancreatic acinar cell) CCK are able to cause the pancreatic responses[5,6].
Oxidative stress has been shown to be involved in the pathophysiology of AP[7,8]. α-Lipoic acid (ALA) is a thiol antioxidant compound with demonstrated direct free-radical scavenging properties[9,10]. However, the effects of ALA on AP have not yet been investigated. Therefore, in this study, we investigated whether ALA can ameliorate the severity of AP using CCK-octapeptide induced AP system.
Male Wistar rats weighing 240-260 g were used. The animals were kept at a constant room temperature of 25 °C with a 12 h light-dark cycle, and allowed free access to water and standard laboratory chow. The rats were fasted for 16 h before the induction of AP. In each experimental group five rats were used.
Avidin-peroxidase and 2-AZINO-bis (3-ethylbenzithiazoline 6-sulfonic acid) tablet substrate were purchased from Sigma (St. Louis, MO, USA). Anti-rat TNF-α, IL-1β and IL-6 antibodies was purchased from R&D Systems (Minneapolis, MN, USA).
ALA at 1 mg/kg was intraperitoneally injected, followed by CCK injected subcutaneously at 75 μg/kg thrice after 1, 3, and 5 h. This whole procedure was repeated for 5 d (n = 5). Other rodents (n = 5) received saline as control. The animals were killed by exanguinations through the abdominal aorta 12 h, after the last CCK injection. The pancrease was quickly removed, cleaned from fat and lymph nodes, weighed, and frozen at -70 °C until use. Rats were treated in accordance with the current law and NIH Guide for Care and Use of Laboratory Animals.
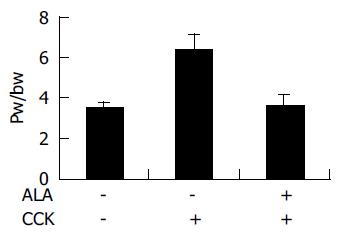
This ratio (pancreatic weight g/body weight g 1 000) was utilized to evaluate the degree of pancreatic edema.
ELISA for IL-6 and TNF-α was carried out in duplicate in 96-well plates (Nunc, Denmark) coated with each of 100 μL aliquots of anti-rat IL-6, IL-1β and TNF-α mAb at 1.0 μg/mL in PBS at pH 7.4 and was incubated overnight at 4 °C. The plates were washed in PBS containing 0.05% Tween-20 (Sigma, St. Louis, MO, USA) and blocked with PBS containing 1% BSA, 5% sucrose and 0.05% NaN3 for 1 h. After additional washes, standards were added and incubated at 37 °C for 2 h. After 2 h incubation at 37 °C, the wells were washed and then each of 0.2 μg/mL of biotinylated anti-rat IL-6, IL-1β, and TNF-α were added and again incubated at 37 °C for 2 h. After the wells were washed, avidin-peroxidase was added and plates were incubated for 20 min at 37 °C. Wells were again washed and ABTS substrate was added. Color development was measured at 405 nm using an automated microplate ELISA reader. A standard curve was run on each assay plate using recombinant IL-6, IL-1β and TNF-α in serial dilutions.
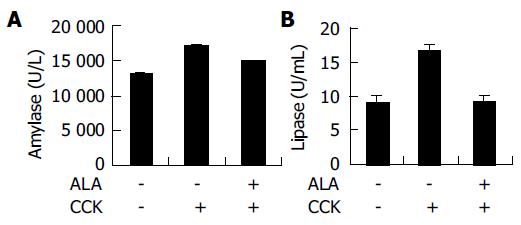
Serum amylase was measured by using an ADIVA 1650 (BAYER, USA). Serum lipase was measured by using a Cobas-mira (Roche, USA).
Results are expressed as mean±SE. The significance of changes was evaluated using Students抯 t-test. Differences between the experimental groups were evaluated by using analysis of variance. Values of P<0.05 were accepted as significant.
To assess the effect of ALA on the pancreatic weight/body weight ratio, pancreatic weight was divided by the body weight of the rats. As shown in Figure 1, in ALA treated group, pancreatic weight/body weight ratio (3.676±0.63) was significantly decreased compared to the DMSO treated group (6.46±0.66, P<0.05, Figure 1).
Secretion of pro-inflammatory cytokines to serum were increased during CCK-induced AP. Then, we investigated whether ALA reduces the serum levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. ALA pre-treatment did not change the level of IL-1β, IL-6 and TNF-α production during CCK-induced AP (Table 1).
| Treatment | IL-1β | IL-6(pg/mL) | TNF-α | |
| CCK | ALA | |||
| - | - | 165.0±26 | 61±3 | 140±51 |
| + | - | 334.7±15 | 85±4 | 289±9 |
| + | + | 334.7±14 | 83±5 | 238±25 |
The levels of serum amylase and lipase are commonly used as a marker of AP. Pre-treatment of ALA significantly decreased the serum amylase and lipase activity CCK -induced AP (Figure 2).
Our findings showed that ALA reduced CCK-induced AP. Furthermore, we showed that pancreatic weight/body weight ratio and serum amylase and lipase were not correlated with the level of cytokines in the rat model. Many previous reports have suggested that ROS may play an important role in the initiation and development of pancreatitis[11,12]. Anti-oxidant, N-acetyl cysteine (NAC) reduced the severity of AP[13,14]. ALA is also a potent anti-oxidant and has anti-inflammatory effect[9,10]. Therefore, in this study, we investigated the effects of ALA on CCK-induced AP. ALA decreased pancreatic weight/body weight ratio in CCK-induced AP. The levels of amylase and lipase usually rise after the onset of symptoms of acute pancreatitis. Compared with serum amylase, serum lipase rises slightly later and remains elevated longer[4]. We found ALA decreased serum level of amylase, lipase in CCK-induced AP (Figure 2). Furthermore, we also examined several kinds of cytokines. IL-1β, IL-6, and TNF-α levels are not attenuated by ALA after CCK treatment (Table 1). Recent study suggest that COX (Cyclooxygenase)-2 inhibition by selective inhibitor (SC-58125), induced alteration of serum amylase and lipase level but not IL-6 and IL-1 production on Caerulein (CAE) induced AP[15]. In the previous report, ALA markedly inhibited radiation or H2O2-induced COX-2 upregulation[16]. On the basis of this report, ALA maybe ameliorates AP via COX-2 inhibition. However, it is needed to investigate its mechanism whether inhibition of COX-2 is involve in the ALA-mediated decrease of AP. Despite of previous studies which link between IL-6 levels and increased severity of AP, recent studies suggest that IL-6 may have an anti-inflammatory role during pancreatitis[17]. IL-6 KO mice exhibited a more severe pancreatitis after CAE injections than wild type mice[17]. Our results indicate that pro-infl-ammatory cytokine levels are elevated in mice treated with or without ALA despite attenuated pancreatitis. This finding suggests that the stimulus for pro-inflammatory cytokines secretion remains intact; however, it is still unclear whether pro-inflammatory cytokines are mechanistically linked to the amelioration of pancreatitis.
In conclusion, this study showed that ALA pr-treatment ameliorated the severity of CCK induced pancreatitis in rats.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 324] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Koehler DF, Eckfeldt JH, Levitt MD. Diagnostic value of routine isoamylase assay of hyperamylasemic serum. Gastroenterology. 1982;82:887-890. [PubMed] |
| 4. | Smotkin J, Tenner S. Laboratory diagnostic tests in acute pancreatitis. J Clin Gastroenterol. 2002;34:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Beglinger C. Potential role of cholecystokinin in the development of acute pancreatitis. Digestion. 1999;60 Suppl 1:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Tachibana I, Shirohara H, Czako L, Akiyama T, Nakano S, Watanabe N, Hirohata Y, Otsuki M. Role of endogenous cholecystokinin and cholecystokinin-A receptors in the development of acute pancreatitis in rats. Pancreas. 1997;14:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Rau B, Poch B, Gansauge F, Bauer A, Nüssler AK, Nevalainen T, Schoenberg MH, Beger HG. Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage? Ann Surg. 2000;231:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Weber CK, Adler G. From acinar cell damage to systemic inflammatory response: current concepts in pancreatitis. Pancreatology. 2001;1:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Park KG, Kim MJ, Kim HS, Lee SJ, Song DK, Lee IK. Prevention and treatment of macroangiopathy: focusing on oxidative stress. Diabetes Res Clin Pract. 2004;66 Suppl 1:S57-S62. [PubMed] |
| 10. | Atmaca G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med J. 2004;45:776-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Sanfey H, Bulkley GB, Cameron JL. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984;200:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Fu K, Sarras MP, De Lisle RC, Andrews GK. Expression of oxidative stress-responsive genes and cytokine genes during caerulein-induced acute pancreatitis. Am J Physiol. 1997;273:G696-G705. [PubMed] |
| 13. | Kim H, Seo JY, Roh KH, Lim JW, Kim KH. Suppression of NF-kappaB activation and cytokine production by N-acetylcysteine in pancreatic acinar cells. Free Radic Biol Med. 2000;29:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Sevillano S, de Dios I, de la Mano AM, Manso MA. N-acetylcysteine induces beneficial changes in the acinar cell cycle progression in the course of acute pancreatitis. Cell Prolif. 2003;36:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Slogoff MI, Ethridge RT, Rajaraman S, Evers BM. COX-2 inhibition results in alterations in nuclear factor (NF)-kappaB activation but not cytokine production in acute pancreatitis. J Gastrointest Surg. 2004;8:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Li L, Steinauer KK, Dirks AJ, Husbeck B, Gibbs I, Knox SJ. Radiation-induced cyclooxygenase 2 up-regulation is dependent on redox status in prostate cancer cells. Radiat Res. 2003;160:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Cuzzocrea S, Mazzon E, Dugo L, Centorrino T, Ciccolo A, McDonald MC, de Sarro A, Caputi AP, Thiemermann C. Absence of endogenous interleukin-6 enhances the inflammatory response during acute pancreatitis induced by cerulein in mice. Cytokine. 2002;18:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |