Published online Aug 21, 2005. doi: 10.3748/wjg.v11.i31.4848
Revised: January 10, 2005
Accepted: January 15, 2005
Published online: August 21, 2005
AIM: To determine the relative potency and contribution of intestinal nutrients to net gastric accommodative relaxation and conscious perception.
METHODS: In 12 healthy subjects, we randomly tested duodenal loads of lipids and carbohydrates (12 mL administered in 4 min) at various caloric concentrations (0.0125-0.8 kcal/mL) separated by 12-24 min wash-out periods of saline infusion. Maximal gastric relaxation was induced at the end of each experiment by i.v glucagon (5 µg/kg), as reference. The reflex gastric response was measured by a barostat, and symptom perception by a 0-6 score questionnaire.
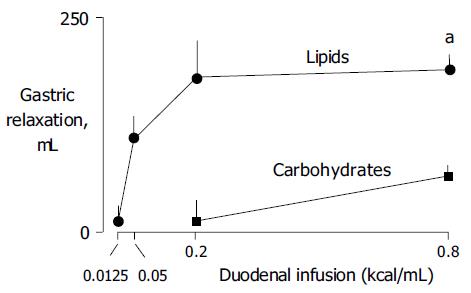
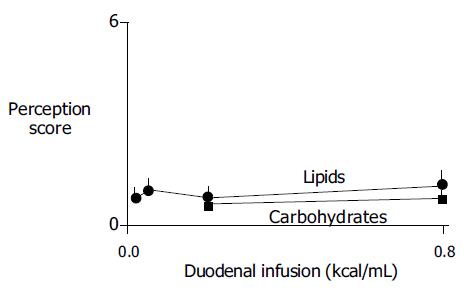
RESULTS: Lipids induced a dose-response gastric relaxation with a steep and early rise. Maximal effect (179±42 mL relaxation) reached at a relatively low concentration (0.2 kcal/mL), maximal lipid-induced relaxation was 61±6% of the glucagon effect. By contrast, duodenal infusion of carbohydrates induced weaker relaxation that became significant only at the high end of the physiological concentration range (65±14 mL with 0.8 kcal/mL). Intestinal nutrient loads, either of lipid or carbohydrates, did not induce significant changes in perception (0.6±0.4 and 0.1±0.4 score increase for the highest concentrations, respectively).
CONCLUSION: Chyme entering the small bowel induces nutrient-specific gastric relaxatory reflexes by a physiologically saturable mechanism. Normally, neither the intestinal nutrient load nor the gastric accommodative response is perceived.
- Citation: Carrasco M, Azpiroz F, Malagelada JR. Modulation of gastric accommodation by duodenal nutrients. World J Gastroenterol 2005; 11(31): 4848-4851
- URL: https://www.wjgnet.com/1007-9327/full/v11/i31/4848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i31.4848
The intestine exerts a feedback control on gastric emptying, and thereby regulates the rate of nutrient delivery into the intestine. The duodenum, in particular, plays a key role in this process, by modulating the level of gastric tone via entero-gastric reflexes. The mechanisms that control the gastric relaxatory response to intestinal nutrients have been extensively studied in experimental animal models[1]. Human data are relatively scarce. Although it has been shown that duodenal nutrients induce a gastric relaxation, and that this effect depends on the type of nutrient[2-4], comparative dose-related responses to various nutrients are not available. Our aim was to compare the relative potency of duodeno-gastric relaxatory reflexes induced physiologically by different types of nutrients, namely, lipids vs carbohydrates in healthy humans employing a combination of enteric nutrient infusion and measurement of gastric tone responses by the barostat.
Twelve healthy subjects without gastrointestinal symptoms (seven women, five men; 20-26 years of age) participated in the study after giving written informed consent. The protocol for the study was previously approved by the Institutional Review Board of the University Hospital Vall d’Hebron.
Using an intra-luminal polyvinyl tube (2 mm outer diameter) located into the duodenum, either saline solution, carbohydrates (Maxijul®; Scientific Hospital Supplies, Barcelona, Spain) or lipids (Intralipid®, Pharmacia and Upjohn, St Cugat del Valles, Spain) were continuously infused at 2 mL/min rate using a perfusion pump (Asid Bonz PP50-300; Lubratronics, Unterschleisshein, Germany). Nutrients were diluted in saline to various caloric concentrations. All perfusates were adjusted to 300 mOsm/L and pH 7.
Gastric tone was continuously measured using a barostat connected by a double lumen tube (127 Argyle; Sherwood Medical, St. Louis, MO, USA) to an intra-gastric bag (1 L capacity, 36 cm maximal diameter, made of ultrathin polyethylene). Tone was measured as changes in intra-gastric volume at a constant pressure level, 2 mmHg above the intra-abdominal pressure. The intra-abdominal pressure was determined at the beginning of each study as the minimal distending pressure that detected respiratory variations, by increasing intrabag pressure in 1 mmHg steps every minute[5]. Detailed description of the barostat has been previously published[5,6].
Subjective perception was measured using a graded questionnaire to measure the intensity and the type of sensations perceived[7]. The graded questionnaire included four graphic rating scales graded from 0 (no perception) to 6 (pain), specifically for scoring the following abdominal sensations: (1) pressure; (2) fullness; (3) nausea; and (4) other type of sensation (to be specified), respectively.
Participants were orally intubated after 6 h fast. The intestinal infusion tube was positioned under fluoroscopic control in the duodenum, 10 cm distal to the pylorus, and the bag of the barostat was introduced into the stomach. The studies were conducted in a quiet, isolated room with participants sitting on an ergonomic chair with the trunk erect.
The barostat was connected to the intra-gastric bag, and gastric tone was continuously recorded on a paper polygraph (model 6006; Letica, Barcelona, Spain). The duodenal tube was connected to the perfusion pump, and saline solution was infused. Nutrient tests were started after 10 min basal recording. In each subject the following nutrient solutions (12 mL administered as 4 mL bolus plus 4 min infusion at 2 mL/min) were tested in random order: (1) lipids at 0.0125, 0.05, 0.2, and 0.8 kcal/mL; and (2) carbohydrates at 0.2 and 0.8 kcal/mL. After each nutrient perfusion, the saline perfusion was restored. In preliminary studies, the duration of the effects of duodenal nutrients on gastric tone depended on the type of nutrient and the caloric load. Hence, to assure the lack of carry-over effects, we adjusted the duration of the saline wash-out periods between nutrients tests, allowing 24 min after the 0.8 kcal/mL lipid dose, 16 min after the 0.2 kcal lipid dose, and 12 min after the rest. At the end of each nutrient perfusion, subjects were asked to fill in the perception questionnaire. After the nutrient tests were completed, glucagon (Glucagon Novo, Laboratorios Novo, Madrid, Spain) was administered as a 5 µg/kg iv bolus, and gastric tone was recorded for another 20 min.
The response of gastric tone to duodenal nutrients was measured as the difference of mean intra-gastric volume recorded by the barostat during the 2 min before and during the last 2 min of each nutrient perfusion. Perception was measured by the highest score (rather than by the mean or cumulative scores) marked on the scales.
Mean±SE of the parameters measured for each nutrient concentration tested was calculated. Comparison of normally distributed data was performed by the paired Student’s t test.
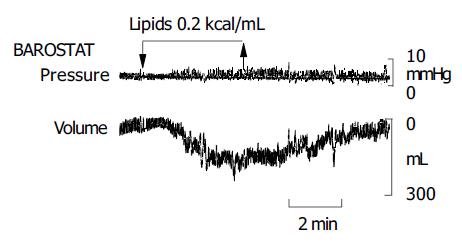
Lipids induced a dose-related gastric relaxation with a steep and early rise (Figure 1). The lowest lipid load (0.0125 kcal/mL) did not modify gastric tone. However, the 0.05 kcal/mL load produced a significant relaxation (P<0.05 vs 0.0125 kcal/mL), exceeding half of the relaxation induced by the maximal lipid concentration (0.8 kcal/mL). A full effect was achieved with a relatively low concentration (0.2 kcal/mL), and no further gastric relaxation was produced by the highest load (0.8 kcal/mL). The maximal effect achieved by lipids was 61±6% of the pharmacological effect of glucagon. The gastric relaxatory response developed rapidly after the lipid administration was started, so that a plateau effect was reached 2 min later (Figure 2). Conversely, during the washout period after the lipid infusion, the relaxation faded and gastric tone reverted to the previous baseline.
Duodenal carbohydrate infusion produced a significantly weaker relaxation than lipids (Figure 1). The 0.2 kcal/mL carbohydrate concentration did not modify gastric tone, though the equicaloric lipid load produced a maximal effect (P<0.05 vs lipids). The highest carbohydrate concentration (0.8 kcal/mL) produced a significant (P<0.05 kcal/mL vs 0.2 kcal/mL) but modest gastric relaxation of only about one-third than that induced by the equicaloric lipid load (P<0.05 vs lipids). The maximal effect achieved by carbohydrates reached only 19% of the glucagon effect.
None of the nutrient loads tested or their reflex effects on gastric tone induced significant conscious perception (Figure 3).
In our study, chyme entering the small bowel induced nutrient-specific gastric relaxatory reflexes by a physiologically saturable mechanism. In healthy subjects, neither the intestinal nutrient load nor the gastric accommodative response was perceived.
As previously shown, lipids in the duodenum induce a gastric relaxatory reflex[2-4]. We have further shown that this effect is concentration-related. Indeed low lipid concentration induced marked effects. Full effect was achieved with a very low concentration, and one fourth of this concentration induced a gastric relaxation which was half of the full effect. This mechanism seems saturable, because a four-fold increase of the full-effect concentration did not increase the magnitude of gastric relaxation. Furthermore, we administered at the end of the study, a pharmacological dose of glucagon to produce a maximal gastric relaxation[8,9], evidenced that the full effect produced by even a very small lipid concentration was about two thirds of the glucagon effect.
By contrast to lipids, intra-duodenal carbohydrates had minor gastric relaxatory effects. Equicaloric amounts of carbohydrates induced significantly smaller gastric relaxation. The same concentration of carbohydrates as that of lipids producing full effect was ineffective, and the maximal concentration tested, achieved only one fifth of the glucagon effect.
The effect of nutrients on gastric tone has been studied on an experimental dog model with isolated intestinal loops[10]. These studies indicate that nutrient specificity depends on the region of intestine stimulated. When infused into the proximal small bowel, lipids are significantly more potent than carbohydrates, which is consistent with that observed in the present study, but in the distal small bowel, carbohydrates elicit a much stronger response. At variance with humans, in this canine model, carbohydrates into the proximal intestine failed to induce a gastric relaxation, but this could be related to the different regions that were exposed. However, in the present study, nutrients were infused directly into the duodenum and a more distal loop was isolated in dogs.
Additional experimental studies in animals have further characterized this nutrient-dependent entero-gastric reflex. In a model of isolated vagus, it was that reflex gastric relaxation is mediated by non-adrenergic, non-cholinergic, vagal fibers[11]. It has also been shown that CCK may be involved in lipid-induced reflexes[12]. However, CCK is only released by long chain fatty acids, but not by the short-medium length acids[13,14]. Furthermore, the rapid onset and reversible response, without appreciable carry-over effects, all point toward a neural rather than a humoral type of response. Hence, the different effects of lipids and carbohydrates seem related to the specific reflex pathways activated.
Detection of the reflex relaxation in the present experiments was possible by an artificial manipulation of intra-gastric conditions. During basal conditions, the stomach is empty and contracted. The barostat applies a small, constant intra-gastric pressure insufficient to expand the gastric walls. However, when the stomach relaxes in response to experimental administration of nutrients directly into the intestine, the intra-gastric volume expands and the barostat makes this relaxation visible by the intra-gastric volume increment. Interestingly, this isobaric expansion does not induce conscious sensations.
It has been shown that intra-gastric volumes are perceived via activation of tension receptors[9], and the present data confirm that expansion and wall elongation do not elicit perception. Nutrients, particularly lipids, have been shown to sensitize mechanoreceptors and heighten perception of gastric distension[2-4], but in the present experimental conditions, nutrients did not modify perception, conceivably because tension receptors were not activated. The gastric expansion measured by the barostat mimics the normal accommodation process, during which gastric filling by food is associated to an accommodative relaxation[1,15]. Indeed, this type of nutrient-mediated reflexes, by modulating the level of contraction/relaxation of the gastric wall, plays a dual role, contributing, both, to the unperceived accommodation of the stomach to the meal, and to the control of the gastric emptying rate. In the present study, even high lipid concentration produced only a partial relaxation, as evidenced by the comparison to the effect of glucagon, suggesting that the stomach still exerts some degree of contraction. This residual contraction, similar to that produced during meal accommodation, is enough to gently force intra-gastric content distally and initiate gastric emptying. Subsequently, the emptying profile is controlled by a net of reflexes depending on the nutrient composition of chyme along the small intestine, to adapt the gastric delivery of nutrients to the intestinal processing capability[1].
The characterization of the nutrient-induced reflexes described in this paper may contribute to a better understanding of the pathophysiology of accommodation-related symptomatic disorders, such as functional dyspepsia. Indeed, it has been shown that dyspeptic patients have impaired entero-gastric reflexes[16,17] that result in impaired gastric accommodation and post-prandial symptoms[18,19], but the specific effects of individual nutrients have not been characterized. Hence, evaluation of the full dose-response to different nutrient elements may help to clarify the reflex dysfunction in functional dyspepsia.
The authors thank Gloria Santaliestra for secretarial assistance.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271-293. [PubMed] |
| 2. | Barbera R, Feinle C, Read NW. Nutrient-specific modulation of gastric mechanosensitivity in patients with functional dyspepsia. Dig Dis Sci. 1995;40:1636-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Feinle C, Grundy D, Read NW. Effects of duodenal nutrients on sensory and motor responses of the human stomach to distension. Am J Physiol. 1997;273:G721-G726. [PubMed] |
| 4. | Feinle C, Grundy D, Fried M. Modulation of gastric distension-induced sensations by small intestinal receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G51-G57. [PubMed] |
| 5. | Azpiroz F, Malagelada JR. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology. 1987;92:934-943. [PubMed] |
| 6. | Azpiroz F, Salvioli B. Barostat measurements. Schuster Atlas of Gastrointestinal Motility in Health and Disease. 2nd Edition ed. Hamilton, Ontario: BC Decker 2002; 151-170. |
| 7. | Azpiroz F. Gastrointestinal perception: pathophysiological implications. Neurogastroenterol Motil. 2002;14:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Notivol R, Coffin B, Azpiroz F, Mearin F, Serra J, Malagelada JR. Gastric tone determines the sensitivity of the stomach to distention. Gastroenterology. 1995;108:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Distrutti E, Azpiroz F, Soldevilla A, Malagelada JR. Gastric wall tension determines perception of gastric distention. Gastroenterology. 1999;116:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Azpiroz F, Malagelada JR. Intestinal control of gastric tone. Am J Physiol. 1985;249:G501-G509. [PubMed] |
| 11. | Azpiroz F, Malagelada JR. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am J Physiol. 1986;251:G727-G735. [PubMed] |
| 12. | Feinle C, D'Amato M, Read NW. Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology. 1996;110:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Feinle C, Rades T, Otto B, Fried M. Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastroenterology. 2001;120:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Lal S, McLaughlin J, Barlow J, D'Amato M, Giacovelli G, Varro A, Dockray GJ, Thompson DG. Cholecystokinin pathways modulate sensations induced by gastric distension in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G72-G79. |
| 15. | Moragas G, Azpiroz F, Pavia J, Malagelada JR. Relations among intragastric pressure, postcibal perception, and gastric emptying. Am J Physiol. 1993;264:G1112-G1117. [PubMed] |
| 16. | Coffin B, Azpiroz F, Guarner F, Malagelada JR. Selective gastric hypersensitivity and reflex hyporeactivity in functional dyspepsia. Gastroenterology. 1994;107:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 152] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Caldarella MP, Azpiroz F, Malagelada JR. Antro-fundic dysfunctions in functional dyspepsia. Gastroenterology. 2003;124:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 410] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 19. | Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |