Published online Aug 21, 2005. doi: 10.3748/wjg.v11.i31.4807
Revised: December 23, 2004
Accepted: December 26, 2004
Published online: August 21, 2005
AIM: Angiotensin II has pro-fibrotic function in the liver. Blockade of the renin-angiotensin-aldosterone-system (RAAS) attenuates hepatic fibrosis. The aim of the present study was to determine the mechanism of angiotensin-converting enzyme inhibitor (ACEI) on the progression of rat hepatic fibrosis.
METHODS: Forty male Wistar rats were divided into three groups. Model group (Mo): The rats were injected subcutaneously with 40% of CCl4 0.25 mL/100 g. Perindopril group (Pe): The rats were injected subcutaneously with 40% of CCl4. Perindopril, equivalent to 2 mg/(kg昫), was administrated. Control group (Nc): the rats were treated with olive oil only. After 4 and 6 wk, the rats were killed. The liver sections were stained with Masson. The protein expressions of AT1R, TGF-β1 and PDGF-BB were examined by Western blot. Nuclear factor κB (NF-κB) DNA binding activity was examined by EMSA (Electrophoretic gel mobility shift assay). Matrix metalloproteinase-2,9 (MMP-2,9) activity was assessed by zymography. Serum laminin (LN) and hyaluronic acid (HA) were measured using radioimmunoassays.
RESULTS: Using Western blot, we clearly provided direct evidence for the expression of AT1R in liver. The expression was up-regulated when fibrogenesis occurred. Perindopril treatment significantly reduced mean fibrosis score, protein levels of AT1R, TGF-β1 and PDGF-BB, serum levels of HA and LN, and the activity of MMP-2,9. NF-κB DNA binding activity markedly increased in model group, perindopril treatment considerably reduced NF-κB DNA binding activity.
CONCLUSION: Perindopril attenuates CCl4-induced hepatic fibrogenesis of rat by inhibiting TGF-β1, PDGF-BB, NF-κB and MMP-2,9
- Citation: Li X, Meng Y, Yang XS, Mi LF, Cai SX. ACEI attenuates the progression of CCl4-induced rat hepatic fibrogenesis by inhibiting TGF-β1, PDGF-BB, NF-κB and MMP-2,9. World J Gastroenterol 2005; 11(31): 4807-4811
- URL: https://www.wjgnet.com/1007-9327/full/v11/i31/4807.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i31.4807
It has been known that renin-angiotensin-aldosterone system (RAAS) plays a key role in the fibrogenesis of local tissue. Angiotensin II (Ang II) exerts local effects on tissue growth and fibrosis by interaction with angiotensin II type 1 receptor (AT1R), leading to a series of pathological changes such as myocardial hypertrophy, renal interstitial fibrosis, and pulmonary fibrosis. In recent years, much attention has been focused on the novel relationship between activation of RAAS and fibrosis of liver. Recent evidence indicates that Ang II may be an important mediator in liver fibrosis. Intra-hepatic RAAS is upregulated in experimental hepatic fibrosis, and serum Ang II levels are frequently elevated in patients with cirrhosis [1,2]. Patients with chronic hepatitis C and a genetic polymorphism associated with increased Ang II synthesis have been reported to develop more severe fibrosis[3].More over, inhibition of Ang II synthesis or the blockade of Ang II type 1 (AT1) receptors markedly reduce fibrosis in experimental models of hepatic fibrosis[4-7]. However, the mechanisms underlying the anti-fibrotic effect of angiotensin-converting enzyme inhibitor (ACEI) on liver fibrosis remain unclear. The present study was undertaken to investigate the effect of ACEI on hepatic fibrosis of rat and the potential mechanism.
Male Wistar rats (250-280 g, n = 40, purchased from Animal Center of the First Military Medical University) were randomly divided into three groups. Model group (Mo): The rats were injected with 40% of CCl4 (the mixture of CCl4 and olive oil) at the dosage of 0.25 mL/100 g subcutaneously thice a week. Perindopril group (Pe): The rats were treated with CCl 4 same to model group, and at the same time, perindopril, equivalent to 2 mg/(kg•d), was given ig. Control group (Nc): the rats were injected with olive oil only.
At the end of the 4 th and 6 th wk, the rats were killed. The tissue of liver was regularly fixed, embed, sliced and stained with Masson. Fibrosis was staged 0-4 based on Scheuer’s scoring system[8] as follows: stage 0: no fibrosis; stage 1: expansion of the portal tracts without linkage; stage 2: portal expansion with portal to portal linkage; stage 3: expansive portal to portal and focal portal to central linkage; and stage 4: cirrhosis.
Serum levels of LN and HA were determined by radioim-munoassays (kit purchased from Northern Biot Co., China) according to the instruction.
Six separate liver tissues from each group were homogenized in 1× cell lysis buffer (Cell Signaling, USA). Fifty micrograms of protein were electrophoresed on 10% or 15% sodium dodecyl sulfate-polyacrylamide under denaturing conditions, and then electrotransferred to PVDF membranes. Nonspecific protein binding was blocked by incubating the membranes with blocking solution (1×TBS, 0.1% Tween-20 with 5% nonfat dry milk) over night at 4 °C. Polyantibody specific for AT1R, TGF-β1 and PDGF-BB (Santa Cruz, USA; 1:700 in 1×TBS containing 0.1% Tween-20 with 5% nonfat dry milk) was applied to the membrane for 2 h at room temperature. After rising with washing buffer (1×TBS, 0.1% Tween-20), HRP-conjugated anti-rabbit IgG antibody (Santa Cruz, USA) diluted at 1:2000 was applied to the membrane for 1 h at room temperature. The detection of specific signal was performed using the Luminol Reagent Solution (Santa Cruz, USA) according to the instruction of the vendor. The protein signal intensity was quantified by a computerized medical image-processing system (GDS-7500, UVP, UK).
The nuclear extracts were prepared either by treating the rats with perindopril for 4 or 6 wk. The nuclear extracts (6 μg) were incubated with 100 pg of 32P-labeled double-stranded nuclear factor κB (NF-κB) oligonucleotide (5’AGTTGAGGGGACTTTCCCAGGC3’; 5’AGTTGCCT-GGGAAAGTCCCCTC 3’) in binding buffer (25 mmol/L Hepes (pH 7.9), 0.5 mmol/L EDTA, 0.5 mmol/L DTT, 1% Nonidet P-40, 5% glycerol, and 50 mmol/L NaCl) containing 2 μg of polydeoxyinosinic deoxycytidylic acid (poly(dI-dC)). The DNA-protein complex was resolved on a native polyacrylamide gel and analyzed by autoradiography. In separate experiments, the nuclear extracts were preincubated with 100-fold excess of unlabeled NF-κB oligonucleotide for 15 min prior to the addition of labeled probe and the samples were further analyzed.
Liver samples were centrifuged at 6 000 r/min for 30 min. Samples were then mixed with an equal volume of 2 non reducing sample buffer, and 50 μg was loaded per well. MMP-2 and MMP-9 were analyzed on gelatin containing gels (7.5% polyacrylamide gel containing 2 mg/mL gelatin). Gels were electrophoresed at 90 V at 4 °C in 1 running buffer. After electrophoresis, SDS was removed from the gel by washing 1 h in 2.5% Triton X-100 solution. This allows the MMPs to renature and digest the surrounding substrate after being incubated overnight at 37 °C in zymogram incubation buffer (50 mmol/L Tris buffer at pH 7.6, 2.5% Triton X-100, 500 mmol/L NaCl, 0.02% NaN3, and 5 mmol/L CaCl2). After incubation, the gel was stained with a solution of 0.25% Coomassie blue R250, 40% methanol, and 10% acetic acid for 2 h at room temperature and destained with 40% methanol, 10% acetic acid untill the bands of lysis became clear.
Analysis of data was performed with oneway ANOVA (SPSS 11.0) and rank sum test. Results were expressed as mean±SD. A value of P<0.05 was regarded as statistical significance.
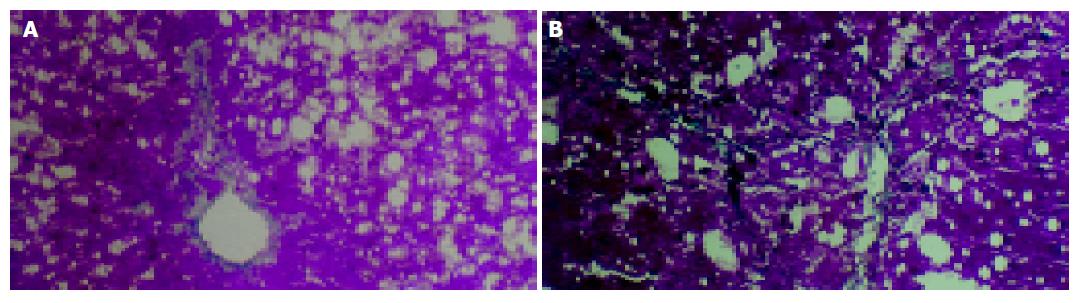
Histologic examination showed that marked and extensive fatty degeneration was present around the central veins and portal tracts except in the control group. At the end of the 4 th wk, in model group, fibroblasts were obviously proliferated in the portal tracts, and collagen was invaded into the hepatic lobules along with the injured limiting laminae. In one case of this group, the formation of pseudolobules could be seen. At the end of the 6th wk, all of the untreated animals had developed severe damage (stage 3 or 4), characterized by extensive portal-portal and portal-central fibrous linkage, distortion of liver architecture, and entrapment of groups of hepatocytes by fibrosis. In contrast, perindopril treatment decreased the score of fibrosis at the end of 4th and 6th wk (P<0.05, Table 1, Figure 1).
| Groups | 4 wk | 6 wk | ||||||||||
| 0 | 1 | 2 | 3 | 4 | n | 0 | 1 | 2 | 3 | 4 | n | |
| Mo | 0 | 1 | 5 | 0 | 1 | 7 | 0 | 0 | 0 | 3 | 3 | 6 |
| Pea,e | 1 | 3 | 2 | 0 | 0 | 6 | 0 | 1 | 1 | 4 | 0 | 6 |
| Nca,c | 6 | 0 | 0 | 0 | 0 | 6 | 6 | 0 | 0 | 0 | 0 | 6 |
Serum LN and HA levels in rats of model group were significantly higher than those of the control group (P<0.05). Treatment with perindopril significantly reduced serum levels of LN and HA (P<0.05, Table 2).
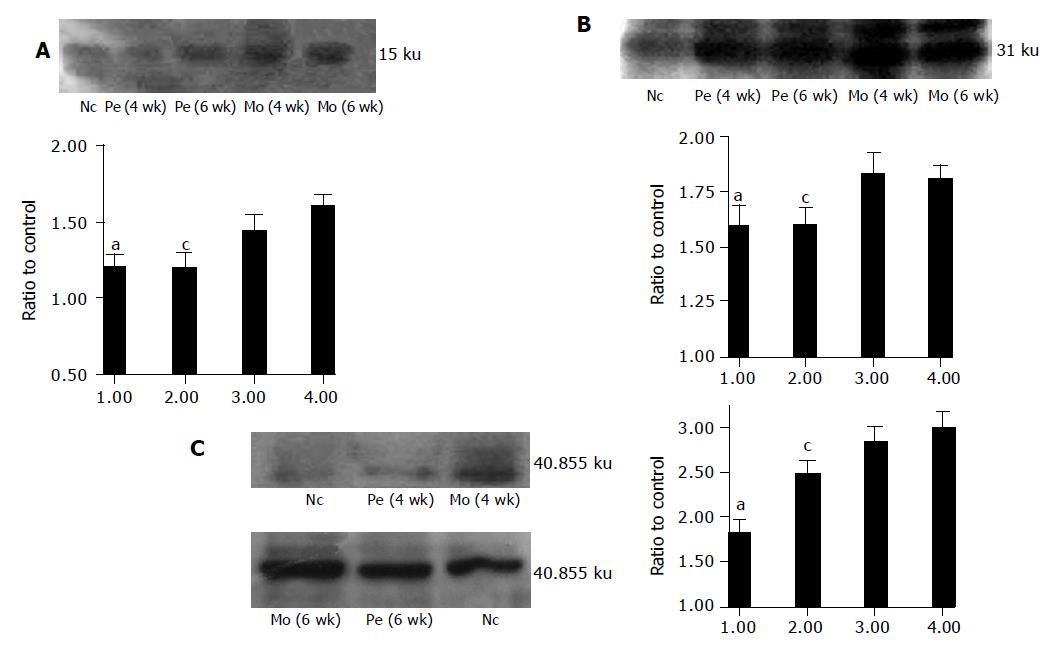
Western blot revealed that AT1R protein expression was upregulated in model group rats compared with control group rats. Perindopril treatment significantly reduced protein levels of AT1R, TGF-β1 and PDGF-BB (Figure 2A-D).
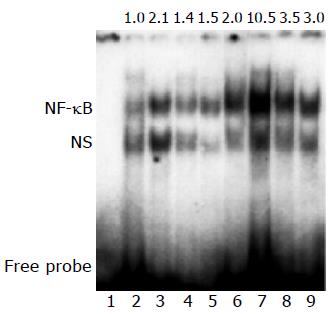
NF-κB DNA binding activity markedly increased in model group, perindopril treatment considerably reduced NF-κB DNA binding activity (Figure 3).
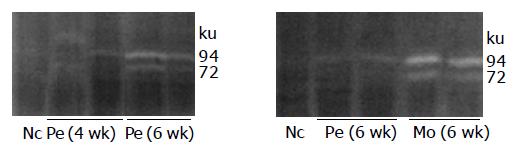
Perindopril treatment considerably reduced the increased MMP-2 and MMP-9 activities that were seen in model group rats (Figure 4).
The RAAS is an important regulator of cardiovascular homeostasis and systemic blood pressure. In recent years, AngII has been shown to have pro-sclerotic actions at many organs including heart, lung and kidney. The RAAS has also been demonstrated in liver with the potential for a functioning intra-hepatic paracrine RAAS. Stimulation of AT1R, belonging to the seven transmembrane, G-protein coupled receptor families, leads to vasoconstriction, fibroblast growth, and aldosterone secretion with synthesis of collagen type I, III, all deem to have a detrimental component in cardiac hypertrophy and organ fibrosis[9,10]. In contrast, ACEI exert effects on inhibiting the growth of cardiac fibroblast and pulmonary fibroblast, resulting in reduction of collagen deposition[11,12]. In vitro studies suggest that AngII may directly stimulate proliferation and enhance bioactivation of TGF-β1, being reduced by RAAS blockade, in cardiac[9] and pulmonary fibroblasts[10].
It is well known that RAAS is always activated in chronic hepatopathy. Powell[3] recently elucidates the inheritance of polymorphisms in the TGF-β1 and angiotensinogen genes and the influence of genotypes on the stage of hepatic fibrosis in patients with chronic hepatitis C. Patients who inherit neither of the pro-fibrogenic genotypes have no or only minimal fibrosis. The documentation raises the novel suggestion that Ang-II may be a mediator of fibrosis in the liver. Hepatic stellate cell (HSC), the main resource of ECM, is the key fibrogenic effector of the liver. The in vitro finding suggest that AT1 receptors are expressed on activated human HSC, and the binding of Ang-II to the receptor induces contraction and proliferation of these cells[13]. Recently, our previous study[14-16] demonstrated that aldosterone synthase gene-CYP11B2 expression was upregulated in HSC when liver fibrosis occurred, and antisterone could partly exert a fibrogenesis-inhibiting effect in the early stage of liver fibrosis. Therefore, RAAS indeed plays a pivotal role in liver fibrosis development through activation of HSC.
In the present study, we examined the in vivo effect of perindopril on hepatic fibrosis of rat. Administration of perindopril can partly alleviate hepatic fibrosis. The fibrosis score and serum fibrosis markers are suppressed by perindopril treatment. Moreover, using Western blot, we clearly provided direct evidence for the expression of AT1R in rat liver. The expression was up-regulated when fibrogenesis occurred. The protein levels of AT1R were suppressed by perindopril. Furthermore, perindopril treatment can inhibit the secretion of Angiotensin II-mediated TGF-β1 and PDGF-BB proteins, which are key factors conducive to the synthesis and the deposition of collagen products by autocrine and paracrine action[17,18]. Further studies are required to elucidate the potential mechanism.
NF-κB is a family of transcription factor that have been shown to be involved in gene regulation of cellular processes like inflammation, fibrogenesis, cell proliferation, and apoptosis[19-21]. This family of proteins is particularly interesting, due to its implication for activation of hepatic stellate cell, the key cellular element involved in the development of hepatic fibrosis. This DNA-binding protein binds to the κB sequence. It promotes transcription of cell adhesion molecules, angiotensinogen and varieties of cytokines such as IL-6, IL-8, TNF-α, which promote hepatic fibrogenesis[22-24]. The present study firstly showed that perindopril treatment significantly reduced NF-κB DNA binding activity in fibrotic liver induced by CCl4. Consequently, perindopril attenuate hepatic fibrosis by inhibiting NF-κB DNA binding activity.
Matrix metalloproteinases (MMPs) degrade the extracellular matrix (ECM) and play critical roles in tissue repair and fibrogenesis. In human chronic liver diseases, there is a upregulation of MMP-2 and MMP-9, which results in increased degradation of basement membrane collagen. Previous study[25] showed that captopril can inhibit MMP-2 and MMP-9 by interacting with the zinc at their active sites, which contribute to the relief of fibrosis. In the current study, MMP-2 and MMP-9 were significantly suppressed by perindopril. In consideration of the action of NF-κB mediated- induction of MMP-2[26], we may demonstrate that perindopril attenuated MMP-2/9 level by inhibiting NF-κB DNA binding activity.
Consequently, the dual effects of ACEI to inhibit NF-κB activity and MMP-2/9, in addition to reducing protein expression of TGF-β1 and PDGF-BB, are likely to contribute to the anti-fibrotic actions of these agents. These findings are in accordance with recent experimental studies that have demonstrated anti-fibrotic effects of RAAS blockade in liver[27].
The effect of existing anti-fibrotic medicines such as interferon and colchicine is limited. However, ACEI have been proven to be an effective anti-fibrotic drug with low incidence of serious side effects. Thus, these medicines may be suitable agents for trials in human chronic liver diseases associated with progressive fibrosis. The present study provides a novel pathway for attenuation of liver fibrosis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Asbert M, Jiménez W, Gaya J, Ginés P, Arroyo V, Rivera F, Rodés J. Assessment of the renin-angiotensin system in cirrhotic patients. Comparison between plasma renin activity and direct measurement of immunoreactive renin. J Hepatol. 1992;15:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Powell EE, Edwards-Smith CJ, Hay JL, Clouston AD, Crawford DH, Shorthouse C, Purdie DM, Jonsson JR. Host genetic factors influence disease progression in chronic hepatitis C. Hepatology. 2000;31:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 305] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Ramalho LN, Ramalho FS, Zucoloto S, Castro-e-Silva Júnior O, Corrêa FM, Elias Júnior J, Magalhães JF. Effect of losartan, an angiotensin II antagonist, on secondary biliary cirrhosis. Hepatogastroenterology. 2002;49:1499-1502. [PubMed] |
| 5. | Paizis G, Gilbert RE, Cooper ME, Murthi P, Schembri JM, Wu LL, Rumble JR, Kelly DJ, Tikellis C, Cox A. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol. 2001;35:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Yoshiji H, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, Nakatani T, Imazu H, Yanase K, Kuriyama S, Fukui H. Inhibition of renin-angiotensin system attenuates liver enzyme-altered preneoplastic lesions and fibrosis development in rats. J Hepatol. 2002;37:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, Vuillemin E, Gallois Y, Douay O, Chappard D. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol. 2002;37:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1198] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 9. | Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 255] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161:1999-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Yokota S, Tsuji H, Kato K. Localization of cathepsin D in rat liver. Immunocytochemical study using post-embedding immunoenzyme and protein A-gold techniques. Histochemistry. 1985;82:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Klickstein LB, Kaempfer CE, Wintroub BU. The granulocyte-angiotensin system. Angiotensin I-converting activity of cathepsin G. J Biol Chem. 1982;257:15042-15046. [PubMed] |
| 13. | Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodés J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 350] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Li X, Meng Y, Yang XS, Wu PS, Li SM, Lai WY. CYP11B2 expression in HSCs and its effect on hepatic fibrogenesis. World J Gastroenterol. 2000;6:885-887. [PubMed] |
| 15. | Yang X, Li X, Wu P, Meng Y, Li S, Lai W. CYP11B2 expression in rat liver and the effect of spironolactone on hepatic fibrogenesis. Horm Res. 2000;53:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Li X, Yang X, Wu P, Meng Y, Li S, Lai W. Gene-CYP11B2 expression in rat liver in hepatic fibrogenesis induced by CCl4. Chin Med J (Engl). 2001;114:64-68. [PubMed] |
| 17. | Kim S, Zhan Y, Izumi Y, Yasumoto H, Yano M, Iwao H. In vivo activation of rat aortic platelet-derived growth factor and epidermal growth factor receptors by angiotensin II and hypertension. Arterioscler Thromb Vasc Biol. 2000;20:2539-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Hamaguchi A, Kim S, Izumi Y, Zhan Y, Yamanaka S, Iwao H. Contribution of extracellular signal-regulated kinase to angiotensin II-induced transforming growth factor-beta1 expression in vascular smooth muscle cells. Hypertension. 1999;34:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Hellerbrand C, Jobin C, Licato LL, Sartor RB, Brenner DA. Cytokines induce NF-kappaB in activated but not in quiescent rat hepatic stellate cells. Am J Physiol. 1998;275:G269-G278. [PubMed] |
| 20. | Lang A, Schoonhoven R, Tuvia S, Brenner DA, Rippe RA. Nuclear factor kappaB in proliferation, activation, and apoptosis in rat hepatic stellate cells. J Hepatol. 2000;33:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Taub R. Blocking NF-kappaB in the liver: the good and bad news. Hepatology. 1998;27:1445-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 663] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10:1498-1506. [PubMed] |
| 24. | Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853-6866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3116] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 25. | Sorbi D, Fadly M, Hicks R, Alexander S, Arbeit L. Captopril inhibits the 72 kDa and 92 kDa matrix metalloproteinases. Kidney Int. 1993;44:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276:44926-44935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |