Published online Aug 14, 2005. doi: 10.3748/wjg.v11.i30.4715
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: August 14, 2005
AIM: To develop a serum or histological marker for early discovery of gastric atrophy or intestinal metaplasia.
METHODS: This study enrolled 44 patients with gastric adenocarcinoma, 52 patients with duodenal ulcer, 14 patients with gastric ulcer and 42 consecutive healthy adults as controls. Each patient received an endoscopy and five biopsy samples were obtained. The degrees of histological parameters of gastritis were categorized following the Updated Sydney System. Anti-parietal cell antibodies (APCA) and anti-Helicobacter pylori (H pylori) antibodies (AHPA) were analyzed by immunoassays. H pylori infection was diagnosed by rapid urease test and histological examination.
RESULTS: Patients with gastric cancer and gastric ulcer are significantly older than healthy subjects, while also displaying higher frequency of APCA than healthy controls. Patients with positive APCA showed higher scores in gastric atrophy and intestinal metaplasia of corpus than patients with negative APCA. Patients with positive AHPA had higher scores in gastric atrophy, intestinal metaplasia, and gastric inflammation of antrum than those patients with negative AHPA. Elderly patients had greater prevalence rates of APCA. Following multivariant logistic regression analysis, the only significant risk factor for antral atrophy is positive AHPA, while that for corpus atrophy is positive APCA.
CONCLUSION: The existence of positive APCA correlates with glandular atrophy in corpus and the presence of positive AHPA correlates with glandular atrophy in antrum. The existence of serum APCA and AHPA betokens glandular atrophy and requires further examination for gastric cancer.
-
Citation: Lo CC, Hsu PI, Lo GH, Lai KH, Tseng HH, Lin CK, Chan HH, Tsai WL, Chen WC, Peng NJ. Implications of anti-parietal cell antibodies and anti-
Helicobacter pylori antibodies in histological gastritis and patient outcome. World J Gastroenterol 2005; 11(30): 4715-4720 - URL: https://www.wjgnet.com/1007-9327/full/v11/i30/4715.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i30.4715
Statistics from the American Cancer Society indicated around 22 000 new cases of gastric carcinoma in the USA for 2001[1]. Gastric cancer, a disease with high mortality, is the second leading cause of cancer death worldwide[2]. An important factor concerning the high mortality rate is the high frequency of advanced gastric cancer at diagnosis. Early diagnosis is difficult because gastric cancer tends to manifest initially with non-specific symptoms and signs.
Helicobacter pylori (H pylori) was categorized as class I carcinogen of gastric cancer by the International Agency for Research on Cancer in 1994[3]. However, most patients with H pylori gastritis are clinically silent and only a fraction of them will develop gastric cancer[4]. Which histological elements would raise the risk of gastric cancer is disputable. Chronic atrophic gastritis was reported in 80-90% and intestinal metaplasia appeared in 70% of patients with gastric carcinoma[5]. Glandular atrophy and intestinal metaplasia are now considered as risk factors for gastric cancer. The identification of both conditions, however, demands invasive procedures and biopsy. To develop a non-invasive, diagnostic tool is an important challenge to all gastroenterologists.
There are no sufficiently sensitive serum markers to enable an early diagnosis of gastric cancer[6]. A low serum pepsinogen I and raised serum gastrin levels were found in patients with gastric cancer[7,8]. However, they lack adequate sensitivity and specificity. The levels of anti-parietal cell antibody (APCA) expression were associated with the histological degree of atrophy[9]. The presence of APCA may represent an early marker of gastric atrophy.
This study attempted to assess differences in histological parameters of gastritis among patients with gastric cancer and other controls. The effectiveness of serum APCA and anti-H pylori antibodies (AHPA) in predicting glandular atrophy and even gastric cancer was also assessed.
This study enrolled 152 consecutive subjects with epigastric discomfort between July 2002 and June 2003. The subjects comprised 44 patients with histologically documented gastric adenocarcinoma, 52 patients with duodenal ulcer, 14 patients with gastric ulcer, and 42 consecutive healthy adults as controls. Those subjects with history of major systemic diseases including diabetes mellitus, adrenal insufficiency, iron deficiency anemia, thyrotoxicosis, myxedema, and Hashimoto’s thyroiditis were excluded.
All subjects were recruited at our hospital and gave informed consent for endoscopic biopsies. Biopsies were executed with jumbo forceps from cancer and non-cancer sites. At least six specimens were obtained from the neoplastic lesions for histological verification. Only those who were histologically documented as gastric adenocarcinoma were included in this study.
In addition, five specimens were collected from antrum and corpus following the standard protocol. These five specimens were classified with a visual analog scale proposed by the Updated Sydney System[10]. This study was approved by the Human Medical Research Committee of the Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
A standardized biopsy protocol was done in all subjects. All subjects underwent endoscopic biopsies and five specimens were extracted from A3 (lesser curvature site of angularis), A1 (lesser curvature site of antrum), A4 (greater curvature site of antrum), B5 (lesser curvature site of mid-body), and B6 (greater curvature site of mid-body). Only cases from whom all five specimens were available were included in this study.
The specimens for histological examinations were fixed in 10% buffered formalin, embedded in paraffin, and sectioned. The sections were stained with a hematoxylin and eosin stain and a modified Giemsa stain[11,12]. The biopsied specimens were assessed by a histopathologist who was unaware of the endoscopic features and clinical data. The morphological variables, including H pylori density, neutrophils (AIS: acute inflammatory score), monocytes (CIS: chronic inflammatory score), lymphoid follicles, glandular atrophy and intestinal metaplasia, were graded with a visual analog scale according to the Updated Sydney System. The scores of all histological parameters in antrum were calculated from means of A1 and A4 and those in corpus from B5 and B6.
The rapid urease test was performed according to our previous studies[13]. Each biopsied specimen was placed immediately in 1 mL of a 10% solution of urea in deionized water (pH 6.8) to which two drops of 1% phenol red solution had been added and incubated at 37°C for up to 24 h. If the yellowish color around the area of inserted specimen changed to bright pink within the 24-h limit, the urease test was considered positive. In our laboratory, the sensitivity and specificity of the rapid urease test were 96% and 91%, respectively[14].
Complete medical history and demographic data was gathered from each patient, comprising age, sex, blood type, residence area, marital status, cigarette and alcohol consumption, beverage of tea or coffee, and drug history.
The presence of H pylori infection was defined as both positivity of rapid urease test and histology.
Serum Anti-parietal cell antibodies was determined with indirect fluorescent antibody tests employing the commercial kit FLUORO-KITTM (DiaSorin Inc. Stillwater, USA) test systems utilizing rat stomach for detection and differentiation of circulating autoantibodies in human serum. Patient’s serum samples were diluted in phosphate buffered saline and overlaid onto tissue cryostat sections fixed on a microscope slide. If APCA appeared in patients’ serum, stable antigen-antibody complexes would be formed. The complexes bound fluorescein labeled anti-human immunoglobulin. The consequent positive reaction, observed with a properly equipped fluorescence microscope, appeared as apple green fluorescence.
Immunoglobulin G (IgG) antibodies to H pylori were measured with an inhouse enzyme immunoassay (EIA). The antigen employed was an acid glycine extract from H pylori strain NCTC 11637. Absorbance readings were converted to reciprocals of the end point titers.
Statistical tests were performed with SPSS system. The χ2 test or the Fisher exact test was used for nominal scale and between groups. Two independent samples were compared by the Student test or the Mann-Whitney/Wilcoxon rank sum test. The stepwise logistic regression analysis was performed with various items and a P value < 0.05 was considered to be significant.
The demographic data for all groups of patients are shown in Table 1. The mean age of patients with gastric cancer (68.8 ± 13.7) and gastric ulcer (71.6 ± 10.7) was significantly higher than that in healthy controls (48.2 ± 13.3). Males had distinctively higher rates of gastric cancers than females. (84% vs 45%, P < 0.05) Patients with gastric ulcer had greater rates of smoking than controls. (71% vs 29%, P < 0.05) No significant differences emerged in other demographic variables between patients with gastric cancer and controls.
| 1HC(n = 42, %) | 2DU(n = 52, %) | 3GU(n = 14, %) | 4GC(n = 44, %) | |
| Age (mean±SD) (yr) | 48.2 ± 13.3 | 51.2 ± 14.3 | 71.6 ± 10.7a | 68.8 ± 13.7a |
| Sex: male | 19 (45) | 34 (65) | 10 (71) | 37 (84)a |
| Blood type | ||||
| A | 15 (36) | 13 (25) | 6 (42) | 12 (27) |
| B | 11 (26) | 16 (31) | 2 (14) | 11 (25) |
| O | 13 (31) | 21 (40) | 5 (36) | 17 (39) |
| AB | 3 (7) | 2 (4) | 1 (7) | 4 (9) |
| Smoking | 12 (29) | 19 (37) | 10(71)a | 15 (34) |
| Daily alcohol use | 4 (10) | 5 (10) | 4 (29) | 6 (14) |
| Daily coffee use | 6 (14) | 3 (6) | 1 (7) | 2 (5) |
| Daily tea use | 11 (26) | 17 (33) | 5 (36) | 8 (18) |
H pylori infection was defined as, both positivity of rapid urease test and histology. The positive rates of H pylori infection of patients with gastric cancer (53%), gastric ulcer (57%), and duodenal ulcer (75%) were notably higher than in controls (24%, Table 2).
The prevalence rates of Anti-parietal cell antibodies in patients with gastric cancer (70%) and gastric ulcer (79%) were much higher than those of controls (36%, P < 0.05, Table 2). Host and bacterial factors related to the presence of APCA in serum were also evaluated. (Table 3) Old age (≥ 60 years) was the only significant factor correlated with the presence of APCA.
| Anti-parietal cell antibodies | P | |||
| Positive(n = 80, %) | Negative(n = 72, %) | Absolute difference(95%CI) | ||
| Age≥60 (yr) | 48 (60)a | 17 (24) | 0.36 (0.22-0.51) | 0 |
| Sex (male) | 58 (73) | 42 (58) | 0.14 (-0.01-0.29) | 0.07 |
| Blood type | ||||
| A | 23 (29) | 23 (32) | -0.03 (-0.18-0.12) | 0.67 |
| B | 21 (26) | 19 (26) | -0.00 (-0.14-0.14) | 0.99 |
| O | 32 (40) | 26 (36) | 0.04 (-0.12-0.20) | 0.63 |
| AB | 4 (5) | 4 (6) | -0.01 (-0.08-0.07) | 0.88 |
| Smoking | 34 (43) | 22 (31) | 0.12 (-0.03-0.27) | 0.13 |
| Daily alcohol use | 13 (16) | 6 (8) | 0.08 (-0.03-0.19) | 0.14 |
| Daily coffee use | 3 (4) | 9 (13) | -0.09 (-0.17-0.00) | 0.05 |
| Daily tea use | 24 (30) | 17 (24) | 0.06 (-0.08-0.21) | 0.38 |
The presence of Anti-Helicobacter pylori antibodies (AHPA) was detected by serum IgG. The investigation assessed host and bacterial factors associated with the presence of AHPA in serum. (Table 4) No significant difference was found between patients with and without AHPA.
| Anti-H pylori antibodies | P | |||
| Positive(n = 82, %) | Negative(n = 70, %) | Absolute difference(95%CI) | ||
| Age≥60 (yr) | 30 (37) | 35 (50) | -0.13 (-0.29-0.02) | 0.1 |
| Sex (male) | 59 (72) | 41 (58) | 0.13 (-0.02-0.29) | 0.08 |
| Blood type | ||||
| A | 25 (30) | 21 (30) | 0.00 (-0.14-0.15) | 0.95 |
| B | 22 (27) | 18 (26) | 0.01 (-0.13-0.15) | 0.88 |
| O | 31 (38) | 27 (39) | -0.01 (-0.16-0.15) | 0.92 |
| AB | 4 (5) | 4 (6) | -0.01 (-0.08-0.06) | 0.82 |
| Smoking | 31 (38) | 25 (36) | 0.02 (-0.14-0.18) | 0.79 |
| Daily alcohol use | 13 (16) | 6 (9) | 0.07 (-0.03-0.18) | 0.18 |
| Daily coffee use | 9 (11) | 3 (4) | 0.07 (-0.02-0.15) | 0.13 |
| Daily tea use | 24 (29 | 17 (24) | 0.05 (-0.09-0.19) | 0.49 |
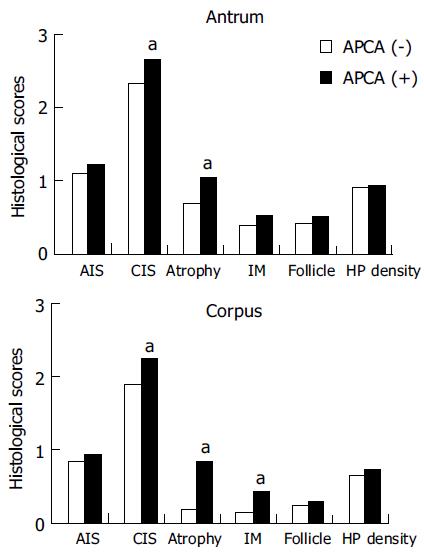
All degrees of histological parameters of gastritis were graded with a visual analog scale according to the Updated Sydney System. The correlation between APCA and histological gastritis is shown in Figure 1. For the histological parameters in antrum, the scores of CIS and atrophy were significantly higher in patients with positive APCA than in those with negative APCA. For the parameters in corpus, the scores of CIS, glandular atrophy, and intestinal metaplasia were significantly higher in patients with positive APCA than in those without. There were no significant differences in scores of other parameters (Figure 1).
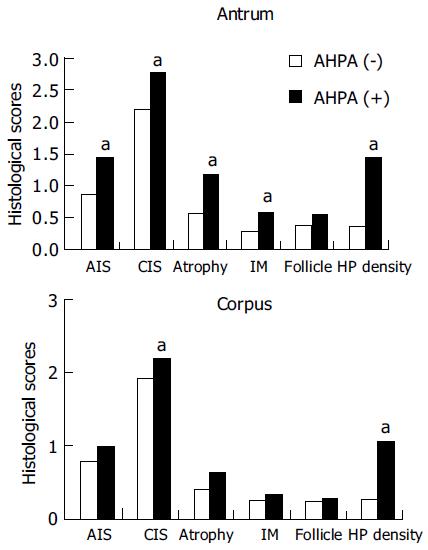
The correlation between AHPA and histological gastritis is shown in Figure 2. For the histological parameters in antrum, the scores for AIS, CIS, glandular atrophy, intestinal metaplasia, and H pylori density were significantly higher in patients with positive AHPA than in those without. (P < 0.05) For parameters in corpus, the scores of CIS and H pylori density were significantly higher in patients with positive AHPA than in those without. There were no significant differences in other parameters (Figure 2).
| Variables | Exp(B) | SE | Wald | Sig |
| Age (yr) | 1.0003 | 0.0165 | 0.0004 | 0.98 |
| Sex | 0.8724 | 0.5639 | 0.0586 | 0.81 |
| Blood_A | 0.6916 | 0.5468 | 0.4547 | 0.50 |
| Blood_B | 0.5688 | 0.5129 | 1.2106 | 0.27 |
| Blood_O | 0.6418 | 0.5042 | 0.7736 | 0.38 |
| Blood_AB | 1.1187 | 0.3705 | 0.0917 | 0.76 |
| Smoking | 1.0790 | 0.5127 | 0.022 | 0.88 |
| Alcohol | 2.0850 | 0.6279 | 1.3694 | 0.24 |
| Coffee | 1.3520 | 0.8278 | 0.1328 | 0.72 |
| Tea | 0.9952 | 0.488 | 0.0001 | 0.99 |
| 1AHPA | 2.8104 | 0.4768 | 4.6965 | 0.03 |
| 2APCA | 1.5004 | 0.4863 | 0.6961 | 0.40 |
| Variables | Exp(B) | SE | Wald | Sig |
| Age (yr) | 1.0272 | 0.0301 | 0.7907 | 0.37 |
| Sex | 3.1120 | 1.2611 | 0.8104 | 0.37 |
| Blood_A | 0.5556 | 0.8903 | 0.4358 | 0.51 |
| Blood_B | 0.5490 | 0.7726 | 0.6023 | 0.44 |
| Blood_O | 0.3659 | 0.8854 | 1.289 | 0.26 |
| Blood_AB | 0.6659 | 0.3478 | 1.3675 | 0.24 |
| Smoking | 1.0528 | 0.8430 | 0.0037 | 0.95 |
| Alcohol | 3.3423 | 0.8884 | 1.8446 | 0.17 |
| Coffee | 0.0003 | 1.4524 | 2.3234 | 0.13 |
| Tea | 3.8250 | 0.8091 | 2.7494 | 0.10 |
| 1AHPA | 1.8250 | 0.8418 | 0.5106 | 0.47 |
| 2APCA | 25.1000 | 1.3096 | 6.0565 | 0.01 |
Multivariant logistic regression analysis of factors affecting severe atrophy (score of glandular atrophy > 2) was also executed. The only factor affecting severe atrophy in antrum was positive AHPA and that in corpus was positive APCA.
Discovery of H pylori led to a widely accepted hypothesis of a multi-step sequence that begins with gastritis and, through atrophy, metaplasia, dysplasia, finally leads to gastric cancer[15]. An odds ratio of 3.6 was reported for gastric cancer among patients infected with H pylori vs those non- infected[16]. The seropositive rate of H pylori infection ranged from 65% to 84% in patients with gastric cancer[17]. In the present study, the infection rate with H pylori was significantly higher in the gastric cancer group (53%) than in controls (24%). (P < 0.05) The positive rate was lower than that reported previously. The reason may relate to the strict policy that presence of H pylori infection was defined as both positivity of rapid urease test and histology.
Glandular atrophy and intestinal metaplasia have been demonstrated to be linked with gastric cancer[5]. An earlier study claimed that in patients with intestinal-type early gastric cancer, more severe glandular atrophy and intestinal metaplasia was noted in all biopsy sites of the stomach[18]. However, another study revealed that both glandular atrophy and intestinal metaplasia were more severe in distal stomach[19,20]. The present study disclosed that scores for glandular atrophy were statistically higher in antrum and corpus of patients with gastric cancer. These results imply that if glandular atrophy occurs, either in antrum or corpus, the risk of developing gastric cancer is greater. The investigation also discovered that scores of intestinal metaplasia and CIS in antrum were significantly higher in patients with gastric cancer. Scores of AIS, CIS, glandular atrophy, intestinal metaplasia, and lymphoid follicles were also higher in corpus. All these results imply that severe degrees of CIS, glandular atrophy and intestinal metaplasia may herald the development of gastric cancer.
Strickland and Mackay even reported in 1973 that APCA was associated with chronic atrophic gastritis of corpus[21]. The levels of APCA expression are connected with the histological degree of atrophy[9], whose presence closely paralleled gastric atrophic status. In the present study, the presence of APCA was significantly higher in patients with gastric cancer than among controls. Evaluation of the histological parameters revealed that the scores of CIS, intestinal metaplasia and glandular atrophy were markedly higher in patients with positive APCA, particularly in corpus. Following multivariant logistic regression analysis, positive APCA was the only significant factor affecting severe atrophy in corpus. These results suggest that the presence of APCA represents an early marker for glandular atrophy in corpus and even for the development of gastric cancer.
When assessing host factors related to the presence of APCA, this study identified old age (>60 years) as the only factor for developing APCA. Although host factors have been thought important for the promotion of atrophic gastritis, the factor that actually triggers these changes remains unknown[22]. Results of this study suggest that age may be the factor promoting atrophic changes. However, the small sample size precludes a definite conclusion at this stage.
The correlations between AHPA and histological gastritis indicated that AHPA correlated well with AIS, CIS, glandular atrophy, intestinal metaplasia, and H pylori density in the antrum. (P < 0.05) These correlation results conform to many previous studies[23]. Following multivariant logistic regression analysis, AHPA emerged as the only significant factor affecting severe atrophy in the antrum. The occurrence of AHPA may act as a predictive marker for glandular atrophy in antrum.
Chronic atrophic gastritis is an independent risk factor for gastric cancer. Timely recognition of these patients was difficult without endoscopic biopsy. If any factor predicting gastric atrophy can be found, these high-risk patients may be diagnosed earlier. Patients with gastric cancer were found to have higher prevalence of APCA and AHPA. (Table 2) The existence of positive APCA correlates well with glandular atrophy in corpus, whereas the presence of positive AHPA correlates with atrophy in antrum. The results of the present work imply that both APCA and AHPA can predict glandular atrophy in corpus and antrum. However, this sample size is small and large-scale age-matched prospective studies are awaited to determine the definite correlation between APCA, AHPA and glandular atrophy.
The authors would like to express their deep appreciation to Dr. Chang-Bih Shie, Yuh-Chyi Chou, Chia-Lin Yeh, Chao-Ming Wu, Lung-Chih Cheng, Miss Yu-San Chen for their invaluable support in this study.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | American Cancer Society. Statistics for 2001. Available at: http: //www.cancer.org/eprise/main/docroot/STT/ stt_02001. Accessed March 21 2002; . |
| 2. | Pisani P, Parkin DM, Muñoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev. 1997;6:387-400. [PubMed] |
| 3. | International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. Schistosomes, Liver flukes and Helicobacter pylori. Lyon: International Agency for Research on Cancer 1994; 177-240. |
| 4. | Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 499] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 5. | Kuipers EJ, Klinkenberg-Knol EC, Vandenbroucke-Grauls CM, Appelmelk BJ, Schenk BE, Meuwissen SG. Role of Helicobacter pylori in the pathogenesis of atrophic gastritis. Scand J Gastroenterol Suppl. 1997;223:28-34. [PubMed] |
| 6. | Ellis DJ, Speirs C, Kingston RD, Brookes VS, Leonard J, Dykes PW. Carcinoembryonic antigen levels in advanced gastric carcinoma. Cancer. 1978;42:623-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Nomura AM, Stemmermann GN, Samloff IM. Serum pepsinogen I as a predictor of stomach cancer. Ann Intern Med. 1980;93:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | McGuigan JE, Trudeau WL. Serum and tissue gastrin concentrations in patients with carcinoma of the stomach. Gastroenterology. 1973;64:22-25. [PubMed] |
| 9. | Ito M, Haruma K, Kaya S, Kamada T, Kim S, Sasaki A, Sumii M, Tanaka S, Yoshihara M, Chayama K. Role of anti-parietal cell antibody in Helicobacter pylori-associated atrophic gastritis: evaluation in a country of high prevalence of atrophic gastritis. Scand J Gastroenterol. 2002;37:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3555] [Article Influence: 122.6] [Reference Citation Analysis (3)] |
| 11. | Hsu PI, Lai KH, Tseng HH, Lo GH, Lo CC, Lin CK, Cheng JS, Chan HH, Ku MK, Peng NJ. Eradication of Helicobacter pylori prevents ulcer development in patients with ulcer-like functional dyspepsia. Aliment Pharmacol Ther. 2001;15:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Hsu PI, Lai KH, Tseng HH, Lin CK, Lo GH, Cheng JS, Chan HH, Chen GC, Jou HS, Peng NJ. Risk factors for presentation with bleeding in patients with Helicobacter pylori-related peptic ulcer diseases. J Clin Gastroenterol. 2000;30:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Peng NJ, Lai KH, Liu RS, Lee SC, Tsay DG, Lo CC, Tseng HH, Huang WK, Lo GH, Hsu PI. Endoscopic 13C-urea breath test for the diagnosis of Helicobacter pylori infection. Dig Liver Dis. 2003;35:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Hsu PI, Lai KH, Tseng HH, Liu YC, Yen MY, Lin CK, Lo GH, Huang RL, Huang JS, Cheng JS. Correlation of serum immunoglobulin G Helicobacter pylori antibody titers with histologic and endoscopic findings in patients with dyspepsia. J Clin Gastroenterol. 1997;25:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 496] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 16. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 17. | Talley NJ, Zinsmeister AR, Weaver A, DiMagno EP, Carpenter HA, Perez-Perez GI, Blaser MJ. Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst. 1991;83:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Craanen ME, Dekker W, Blok P, Ferwerda J, Tytgat GN. Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut. 1992;33:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Yoshimura T, Shimoyama T, Fukuda S, Tanaka M, Axon AT, Munakata A. Most gastric cancer occurs on the distal side of the endoscopic atrophic border. Scand J Gastroenterol. 1999;34:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Stolte M, Meining A. Helicobacter pylori gastritis of the gastric carcinoma phenotype: is histology capable of identifying high-risk gastritis? J Gastroenterol. 2000;35 Suppl 12:98-101. [PubMed] |
| 21. | Strickland RG, Mackay IR. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973;18:426-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 334] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Azuma T, Konishi J, Tanaka Y, Hirai M, Ito S, Kato T, Kohli Y. Contribution of HLA-DQA gene to host's response against Helicobacter pylori. Lancet. 1994;343:542-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Fukao A, Komatsu S, Tsubono Y, Hisamichi S, Ohori H, Kizawa T, Ohsato N, Fujino N, Endo N, Iha M. Helicobacter pylori infection and chronic atrophic gastritis among Japanese blood donors: a cross-sectional study. Cancer Causes Control. 1993;4:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |