Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4524
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: August 7, 2005
AIM: To study the changes of endogenous interleukin 18 (IL-18) levels and evaluate the role of IL-18 on lung injury following gut ischemia/reperfusion.
METHODS: A superior mesenteric artery occlusion model was selected for this research. The mice were randomly divided into four groups: Sham operation (sham), ischemia (0.5 h) followed by different times of reperfusion (I/R), and I/R pretreated with exogenous IL-18 (I/R+IL-18) or IL-18 neutralizing antibody (I/R+IL-18Ab) 15 min before ischemia. Serum IL-18 levels were detected by Western blot and ELISA, and the levels of IL-18 in lung tissue were evaluated by immunohistochemical staining. For the study of pulmonary inflammation, the lung myeloperoxidase (MPO) contents and morphological changes were evaluated.
RESULTS: Gut ischemia/reperfusion induced rapid increase of serum IL-18 levels, peaked at 1 h after reperfusion and then declined. The levels of IL-18 in lung tissue were gradually enhanced as the progress of reperfusion. Compared with I/R group, exogenous administration of IL-18 (I/R+IL-18) further remarkably enhanced the pulmonary MPO activity and inflammatory cell infiltration, and in I/R+IL-18Ab group, the content of MPO were significantly reduced and lung inflammation was also decreased.
CONCLUSION: Gut ischemia/reperfusion induces the increase of IL-18 expression, which may make IL-18 act as an important proinflammatory cytokine and contribute to gut ischemia/reperfusion-induced lung inflammation.
- Citation: Yang YJ, Shen Y, Chen SH, Ge XR. Role of interleukin 18 in acute lung inflammation induced by gut ischemia reperfusion. World J Gastroenterol 2005; 11(29): 4524-4529
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4524.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4524
Multiple organ dysfunction syndrome (MODS) is the leading cause of death in critically ill patients. Although systemic inflammation characteristic of MODS can result in damage to any organ, onset of the syndrome is usually heralded by the development of respiratory insufficiency[1,2]. Gut ischemia and reperfusion (I/R) is a prime mechanism that results in the pathogenesis of MODS partly dependent on neutrophils[2]. Neutrophils can be activated in response to reperfusion of ischemia tissue or exposure to endotoxin and pro-inflammatory cytokines[1]. Activated neutrophils adhere to endothelium through adhesion molecules, resulting in localized release of proteases, reactive oxygen species (ROS) and various cytokines and inflammatory mediators that contribute to tissue injury and failure[1,2].
Interleukin 18 (IL-18), known initially as an IFN-γ-inducing factor, is a pleiotropic proinflammatory cytokine and it has direct proinflammatory properties. In this respect, IL-18 induces production of proinflammatory cytokines such as TNF-α, IL-1β and chemokines such as IL-8, MIP-1α and MCP-1[3,4], and upregulates expression of adhesion molecules such as ICAM-1, VCAM-1 on endothelial cells[3,5]. Furthermore, IL-18 is found to activate neutrophils and promote neutrophil migration, adhesion and accumulation in vivo[6]. These data suggest that IL-18 may be involved in the inflammatory injuries associated with I/R[1,2,7,8]. Therefore, recent evidence showing that some local tissue ischemic injuries are associated with increased IL-18 levels[9,10], and elevated IL-18 levels have been detected in response to hepatic I/R injury[11]. A direct correlation has been observed between IL-18 levels and liver neutrophils sequestration and liver injury, which suggests that IL-18 is required for facilitating neutrophil-dependent hepatic I/R injury[11]. Similarly, increased levels of IL-18 have been detected in isolated atrial trabeculae during I/R injury[12]. Inhibition of IL-18 or caspase-1 activity attenuated tissue and circulating levels of IL-18 and improved myocardial contractility, suggesting that endogenous IL-18 play a significant role in I/R-induced human myocardial injury[12]. Furthermore, Melnikov et al[13], reported that caspase-1 and IL-18 play an important role in the ischemic acute renal failure; however, there is also evidence suggesting that activated caspase-1 and its inflammatory products are not crucial to the induction of inflammation after renal I/R[14]. It is intriguing to consider the role of IL-18 in the I/R-induced local organ injury, and no evidence exists on the role of IL-18 in the MODS following I/R. Therefore, the major aim of present study was to determine the change of expression and role of IL-18 in the acute pulmonary injury after gut ischemia/reperfusion.
C57BL/6J mice (SPF) were obtained from animal center, SIBS, Shanghai. For the experiments, 8-10-wk-old mice weighing 22-26 g were used. rmIL-18 was expressed and purified in our lab (purity>95% by SDS-PAGE) and the activity was confirmed by its ability to induce IFN-γ production by mouse spleen cells. For immunohistochemical analysis, polyclonal rabbit anti-mouse IL-18 antibody was purchased from Boster (Wuhan, China). For immunoprecipitation and Western blot analysis, polyclonal rabbit anti-murine IL-18 antibody was provided by PeproTech (Rocky Hill, NJ, USA).
Mice were anesthetized by intraperitoneal injection of urethane (250 mg/mL, 1.25 mg/g wt of mouse, Sigma). The abdomen was rinsed with 75% ethanol, a midline laparotomy was performed and the superior mesenteric artery was occluded with an arterial clamp. Intestinal ischemia was confirmed by pulselessness of the mesenteric artery and paleness of the jejunum and ileum. Sham-operated mice underwent the same procedure, but without vascular occlusion. After 30 min, the clamp was removed, sterile saline was injected into the peritoneal cavity for resuscitation, and the mice were sutured.
The mice were randomly divided into four groups (n = 5 in each): sham, I/R, I/R+IL-18, and I/R+anti-IL-18. For exogenous IL-18 studies, animals were pretreated with either IL-18 (5 µg/mouse) or sterile saline via the lateral tail vein 15 min before ischemia. For IL-18 neutralization studies, mice received monoclonal anti-IL-18 antibody (25 µg/mouse, MBL, Nagoya, Japan) or sterile saline 15 min before ischemia. Animals underwent 30 min of superior mesenteric artery occlusion (or sham operation) followed by indicated periods of reperfusion were killed, lung tissues, and blood samples were taken for analysis. Excised lungs were frozen in liquid nitrogen and stored at -70 °C before determination of myeloperoxidase (MPO) activity. For histological or immun-ohistochemical studies, lungs were immersed in 10% neutral buffered formalin before sectioning. All blood samples were kept at room temperature for 2 h to clot, and serum was removed and stored at -70 °C until the time of assay.
Animals underwent cardiac puncture to obtain blood at the indicated times in the reperfusion period. Systemic levels of IL-18 were evaluated by immunoprecipitation followed by Western blot. Serum was diluted to 1 mL with PBS, and serum IL-18 was immunoprecipitated with rabbit anti-murine IL-18 antibody. Then, the immunoprecipitates were resolved by SDS-PAGE (12-15% acrylamide) under reducing conditions. Gels were transferred to NC membranes and incubated with primary antibodies (1:1 000) at 4 °C overnight. Then mouse anti-rabbit IgG peroxidase was added and developed by ECL. Serum levels of IL-18 were further measured by ELISA according to the manufacturer’s instructions (Boster, Wuhan, China). The detection limit for IL-18 was <5 pg/mL.
MPO activity is an indicator of neutrophil accumulation. The content of MPO in the tissues was measured as previously described with slight modification[7]. The lungs were homogenized for 30 s in 4 mL 50 mmol/L potassium phosphate buffer (pH 6.0) and then centrifuged for 30 min at 16 000 g at 4 °C. The pellet was resuspended in 1.5 mL 50 mmol/L potassium phosphate buffer (pH 6.0) containing 5 g/L cetrimonium bromide. The samples were sonicated for 90 s and then incubated in a 60 °C water bath for 2 h, and centrifuged for 30 min at 35 000 g at 4 °C. The supernatant 50 μL was added to 950 μL of 50 mmol/L potassium phosphate buffer (pH 6.0) containing 0.167 mg/mL o-dianisidine (Sigma) and 5 μL/L hydrogen peroxide. Absorbance of 460 nm was measured at 1 and 3 min. MPO content per gram of wet tissue was calculated as: MPO content (A/g wet tissue) = (A460(3 min)-A460(1 min))/tissue weight (g).
Lungs were immersed in 10% neutral buffered formalin before sectioning. Sections (4 μm) were obtained from paraffin-embedded tissue samples, stained with hematoxylin-eosin (HE), and examined for histological evaluation of tissue damage under a microscope and photographed. For immunohistochemical analyses, paraffin-embedded lung sections of 4 μm were incubated overnight at 4 °C with polyclonal rabbit anti-mouse IL-18 antibody (dilution 1:100). Biotinylated IgG was added as second antibody. Horseradish peroxidase-labeled streptomycin-avidin complex was used to detect second antibody. Slides were stained with diamino-benzidine, counterstained with hematoxylin, and finally examined under light microscope. The brown or dark brown stain was considered as positive.
All data are presented as mean±SD. Significant differences between groups were determined using Student’s t-test. P<0.05 is considered as statistically significant.
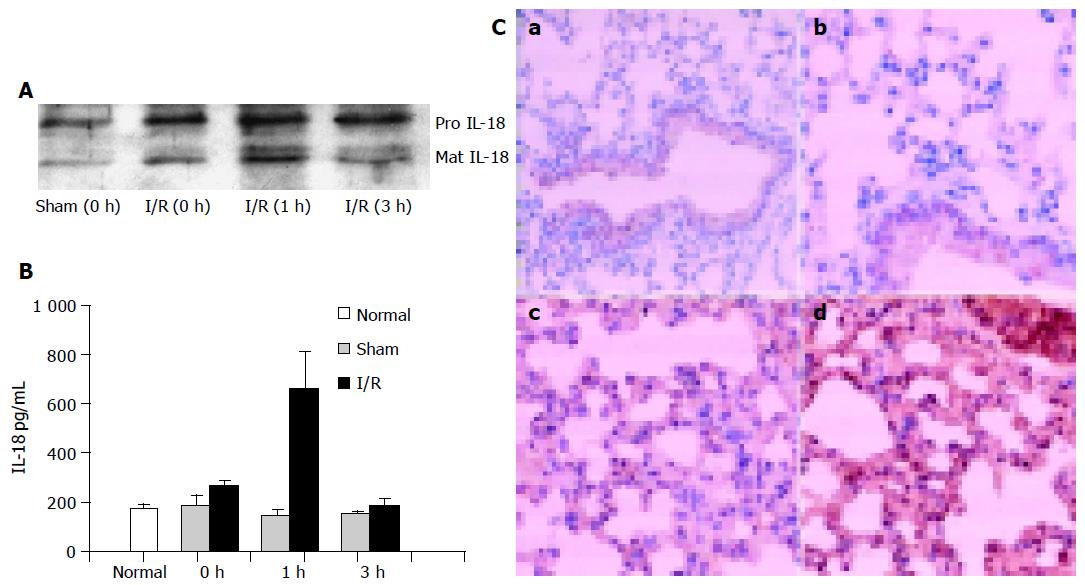
IL-18 has been shown to be upregulated after ischemia in the kidney, heart, and liver. Therefore, we investigated whether the levels of IL-18 changed in response to gut ischemia/reperfusion. The serum, obtained from sham and from gut I/R model at different times of reperfusion, was first evaluated by Western blot, and the change of IL-18 levels is shown in Figure 1A. Two bands were detected in all serum samples, which are consistent with the “pro” and “mature” form of IL-18. At time 0, the content of IL-18 from I/R group, for both pro and mature, was elevated as compared with that from sham-operated mice, and the levels of mature IL-18 (mIL-18) was further considerably elevated 1 h after reperfusion, and then decreased at 3 h (Figure 1A).
Mouse serum IL-18 was further detected by ELISA after gut ischemia/reperfusion or sham operation. As shown in Figure 1B, IL-18 levels from normal mouse were <200 pg/mL. Consistent with the previous results, there was a marked increase in serum IL-18 from I/R group, peak at 1 h (at 660 pg/mL), followed by a decline. In the sham group, there was little measurable change in IL-18 levels during all reperfusion time.
Furthermore, lung tissues obtained from normal mouse and I/R mice with 1 or 3 h reperfusion was examined by immunohistochemistry. Besides the constitutive expression of IL-18 in air way epithelium, normal lung tissue lacked immunoreactive IL-18 (Figure 1C, a and b); during 1-3 h of reperfusion, gradually increased levels of IL-18 were observed with prominent IL-18 staining (Figure 1C, c and d). These data indicated that IL-18 is induced at the early phase of ischemia/reperfusion and may participate in the pulmonary inflammation.
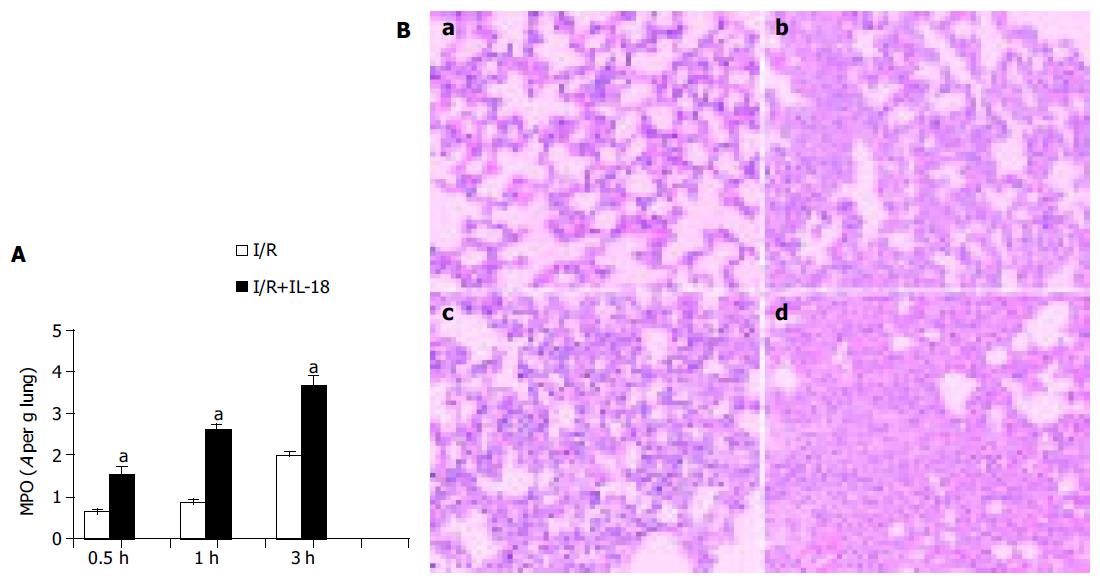
Ischemia-induced expression of IL-18 increased remarkably at the early phase of reperfusion, suggesting that IL-18 may be an early inflammatory mediator and involved in the injurious processes in lung after gut I/R, which prompted us to suppose whether administration of exogenous IL-18 could exert early effects on neutrophil activation and accelerate lung injury. As shown in Figure 2, in the IL-18-nontreated group, gut ischemia/reperfusion induced gradually increased MPO activity in the lungs at 0.5, 1, or 3 h after reperfusion (0.65±0.08, 0.86±0.19, or 1.99±0.17 respectively), and IL-18 injection further dramatically enhanced the MPO activity in the lung tissues at each indicated times after reperfusion: the MPO value in IL-18-treated mice was further increased to 1.54±0.38 (a 237% increase), and 2.63±0.20 (a 306% increase) at 0.5 or 1 h after ischemia respectively, as compared with IL-18-nontreated group (P<0.05); and IL-18 administration induced much more neutrophils infiltration in lung within 1 h of reperfusion than that of 3 h of reperfusion in IL-18-nontreated group (Figure 2A). Furthermore, after 3 h of reperfusion, the MPO activity was further increased to higher level (3.68±0.45, a 184% increase) compared with that from IL-18-nontreated mice (P<0.05). These data suggested that IL-18 injection enhanced pulmonary MPO activity and augmented the neutrophil accumulation in lung.
Lung inflammation in this model was evident from histological analysis (Figure 2B). As previously reported, massive neutrophil infiltration was observed in the lung tissues from gut I/R model within 3 h of reperfusion (Figure 2B, b). Although the infiltration in IL-18-nontreated mice at 1 h after ischemia was not obvious (Figure 2B, a), IL-18 injection significantly enhanced the pulmonary infiltration after 1 h of reperfusion (Figure 2B, c). Furthermore, treatment of IL-18 induced more serious inflammatory cell infiltration compared with that of IL-18-nontreated mice as the reperfusion time extended to 3 h (Figure 2B, b and d), which in accordance with the higher MPO activity in IL-18-treated group. Both indices of lung inflammation, including histological changes and MPO activity, suggest that IL-18 injection may accelerate and augment the lung inflammatory injury in response to gut I/R, and IL-18 may be involved in the lung pathogenesis in this I/R model as an early inflammatory mediator.
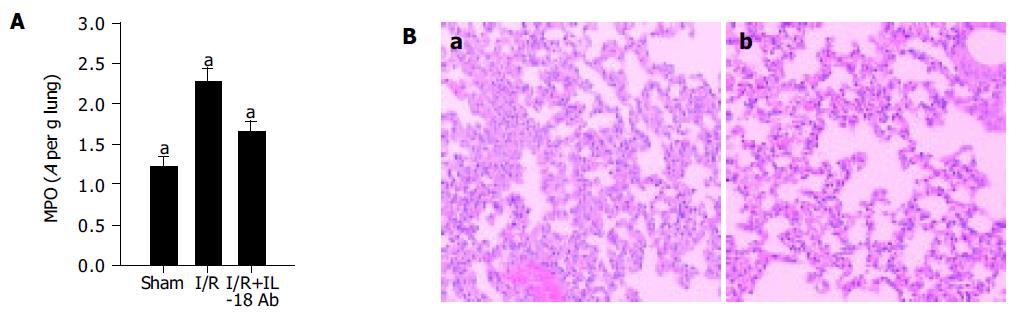
To further determine the role of induced IL-18 in the lung pathogenesis, we investigated whether in vivo neutralization of IL-18 could modify neutrophil-mediated acute inflammatory response induced by gut I/R. Anti-IL-18Ab (25 µg/mouse) was administered 15 min before ischemia insult, and after 3 h of reperfusion, the MPO levels were determined and the lung inflammation was evaluated histopathologically. As shown in Figure 3, anti-IL-18 recipient mice exhibited significantly reduced tissue MPO activity (more than 50% decrease in the lung MPO levels) as compared with the positive control (P<0.05, Figure 3A). Consistent with this, the inflammatory infiltration was significantly reduced in anti-IL-18-treated mice (Figure 3B). These data suggested the IL-18Ab attenuated lung inflammation induced by gut I/R and supported the concept that endogenous IL-18 functioned as an important proinflammatory factor, which may be involved in the neutrophil sequestration and lung injury in this gut I/R model.
To date, IL-18 has been known for its role in infectious inflammatory injury. However, few investigations have been accomplished regarding the role of IL-18 during non-infectious acute inflammatory reactions such as those that occur in ischemia/reperfusion. Previous reports have shown that IL-18 have important regulatory role during organ injury induced by local ischemia/reperfusion[11-13]. In the current study, we used a model of acute lung inflammation induced by gut ischemia/reperfusion, which results in damage of capillary endothelial cells and neutrophil influx, and oxidants, proteases released from inflammatory cells damage lung cells and tissue matrix[1-3]. The role of IL-18 in this model of lung injury has not been explored. The current study provides evidence that endogenous IL-18 may act as a proinflammatory cytokine and contribute to gut ischemia/reperfusion-induced lung inflammation.
Our results, consistent with the previous reports, have shown that local ischemia may induce the elevation of IL-18 level[11-13]. It promptly reached peak at 1 h and then declined to baseline. However, the levels of IL-18 in lung tissue were not the same. Constitutively, expression of IL-18 in airway epithelium has been observed by using immunohistochemistry, and as the reperfusion time extended to 3 h, the level of IL-18 in air way epithelium was greatly enhanced and immunoreactive IL-18 dispersed all over the lung tissue, which suggests that pulmonary expression of IL-18 may be enhanced during reperfusion as the inflammatory agents secreted from intestine reached the lung tissue and further, the IL-18 receptor mediated mechanism may also account for the pulmonary accumulation of IL-18, which in accordance with the rapid decline of serum IL-18 level during reperfusion.
IL-18 has been known to be produced as a proform and stored in cytoplasm. After the cleavage of caspase-1, it turns to a functional, mature protein. By Western blot analysis, the IL-18 mature protein was quickly increased after 1 h of reperfusion (Figure 1A), which suggests that gut ischemia may result in the activation of caspase-1 and enhance the maturation and secretion of IL-18. Furthermore, previous reports have shown that ischemia also induces IL-1β expression. Therefore, it is possible that intestinal caspase-1 may play a key role in expression of both the mature IL-18 and IL-1β, and ischemic gut may serve as a major source for circulating IL-18 in this model.
Experimental studies have suggested that IL-18 protects against bacterial and viral agents[3], which is thought to be attributable in part to the infiltration of inflammatory cells after treatment with IL-18. There is also evidence suggesting that IL-18 may activate neutrophils and promote neutrophil migration and accumulation in vivo[6]. In the current studies, we showed that the in vivo exogenous administration of IL-18 before gut ischemia greatly enhanced neutrophil recruitment as well as lung inflammation (Figure 2). Conversely, IL-18 blockade reduced the evidence of lung inflammation by neutralizing Ab (Figure 3). These results suggest that IL-18 may, like IL-1β, be an important early pro-inflammatory cytokine involved in the pathogenesis of acute lung inflammation induced by gut ischemia/reperfusion. Furthermore, these data also suggest that the preoperative levels of serum IL-18 may represent a valuable parameter to aid the postoperative prognostic determinant in clinical patients with ischemia-induced MODS.
But how IL-18 is activated, and how it enhances the pulmonary inflammation during ischemia/reperfusion is still unknown. There is evidence indicating that NO suppresses IL-18 processing by inhibiting caspase-1 activity[15,16]. It is likely that ischemia-induced decrease of NO levels may result in the activation of caspase-1 and the further secretion of mature IL-18. Furthermore, it is clear that IL-18, a pleiotropic proinflammatory cytokine, has an endogenous role in enhancing production of several early response cytokines during lung inflammation, such as TNF-α, IFN-γ, and IL-1β. Previous reports have shown that IL-18 may activate neutrophil and promote neutrophil migration and accumulation in vivo via a TNF-α-dependent pathway[6,17]. However, the mechanism of pulmonary neutrophils seques-tration is possibly independent of TNF-α in this lung injury model[18], which suggests that the function of IL-18 may not be totally dependent on the role of TNF-α. These works are now in progress in our group.
The present data expand our knowledge of the immun-oregulatory properties of IL-18. Our findings are clinically applicable to ischemia/reperfusion injury during surgical operation such as small intestinal transplantation, and modulation of IL-18 expression may enhance endogenous protective effects, leading to a reduction in organ injury.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 2. | Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 401] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 486] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 436] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 392] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C, Cunha F, Liew FY, McInnes IB. A role for IL-18 in neutrophil activation. J Immunol. 2001;167:2879-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 240] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Netea MG, Fantuzzi G, Kullberg BJ, Stuyt RJ, Pulido EJ, McIntyre RC, Joosten LA, Van der Meer JW, Dinarello CA. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol. 2000;164:2644-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol. 2001;167:7060-7068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Zaremba J, Losy J. Interleukin-18 in acute ischaemic stroke patients. Neurol Sci. 2003;24:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Hedtjärn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22:5910-5919. [PubMed] |
| 11. | Takeuchi D, Yoshidome H, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, Morita Y, Miyazaki M. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004;39:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci USA. 2001;98:2871-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 330] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Daemen MA, Denecker G, van't Veer C, Wolfs TG, Vandenabeele P, Buurman WA. Activated caspase-1 is not a central mediator of inflammation in the course of ischemia-reperfusion. Transplantation. 2001;71:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kozar RA, Holcomb JB, Hassoun HT, Macaitis J, DeSoignie R, Moore FA. Superior mesenteric artery occlusion models shock-induced gut ischemia-reperfusion. J Surg Res. 2004;116:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kim YM, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1beta and IFN-gamma-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1beta-converting enzyme). J Immunol. 1998;161:4122-4128. [PubMed] |
| 17. | Canetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol. 2003;171:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Caty MG, Guice KS, Oldham KT, Remick DG, Kunkel SI. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia-reperfusion injury. Ann Surg. 1990;212:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 198] [Article Influence: 5.7] [Reference Citation Analysis (0)] |