Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4478
Revised: December 1, 2004
Accepted: December 3, 2004
Published online: August 7, 2005
AIM: Type IV collagenase including MMP-2 and -9 plays an important role in cancer cell invasion and metastasis and is an attractive target for mAb-directed therapy. The immunoreactivity of mAb 3G11, a mAb directed against type IV collagenase in human colorectal carcinomas, was studied by immuno-histochemical (IHC) staining. mAb 3G11 was conjugated to an antitumor antibiotic lidamycin (LDM). The antitumor activity of 3G11-LDM conjugate against colon carcinoma was investigated in mice.
METHODS: ELISA, gelatin zymography, and Western blot assay were used for the biological characterization of mAb 3G11. The immunoreactivity of mAb 3G11 with human colorectal carcinomas was detected by IHC staining. The cytotoxicity of LDM and 3G11-LDM conjugate to human colon carcinoma HT-29 cells was examined by clonogenic assay and MTT assay. The therapeutic effect of conjugate 3G11-LDM was evaluated with colon carcinoma 26 in mice.
RESULTS: As shown in ELISA, mAb 3G11 reacted specifically with type IV collagenase, while 3G11-LDM conjugate also recognized specifically its respective antigen. In IHC assay, mAb 3G11 showed positive immunoreactivity in most cases of colorectal carcinoma, and negative immunoreactivity in the adjacent non-malignant tissues. By gelatin zymography, the inhibition effect of mAb 3G11 on the secretion activity of type IV collagenase was proved. In terms of IC50 values in MTT assay, the cytotoxicity of LDM to human colon carcinoma HT-29 cells was 10 000-fold more potent than that of mitomycin C (MMC) and adriamycin (ADM). 3G11-LDM conjugate also displayed extremely potent cytotoxicity to human colon carcinoma HT-29 cells with an IC50 value of 5.610-19 mol/L. 3G11-LDM conjugate at the doses of 0.05 and 0.1 mg/kg inhibited the growth of colon carcinoma 26 in mice by 70.3 and 81.2%, respectively.
CONCLUSION: mAb 3G11 is immunoreactive with human colorectal carcinoma and its conjugate with LDM is highly effective against colon carcinoma in mice.
- Citation: Li L, Huang YH, Li Y, Wang FQ, Shang BY, Zhen YS. Antitumor activity of anti-type IV collagenase monoclonal antibody and its lidamycin conjugate against colon carcinoma. World J Gastroenterol 2005; 11(29): 4478-4483
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4478
There were 622 000 deaths of colorectal cancer globally in 2002 according to the World Health Report 2004 of WHO. Colorectal cancer can be considered as a complex disease, with a combination of predisposing genetic variants and environmental factors that contribute to the illness as a whole. Since colorectal carcinoma is a leading cause of cancer death in the world, many therapeutic strategies are being investigated, among which the recent major achievements are Avastin and Erbitux, as antibody therapeutics approved for treatment of refractory and advanced colorectal carcinoma by the FDA of USA in 2004. This indicates that antibody-based drugs are promising for colorectal cancer therapy.
Lidamycin (LDM), also called C-1027, is a member of enediyne antitumor antibiotics family and binds to the minor groove of DNA, causing double-strand breaks and apoptosis. LDM consists of an apoprotein (LDP) of 10.5 ku and an enediyne chromophore (LDC) of 843 Da. These two parts of the molecule, connected each other through non-covalent binding, can be dissociated and reconstituted[1,2]. Because of its extremely potent cytotoxicity against cancer cells and its remarkable activity of anti-angiogenesis and anti-metastasis, LDM can serve as an “effector” agent or “warhead” molecule to construct immunoconjugates[3-5]. In addition, as a potential chemotherapeutic agent for cancer treatment, LDM has recently entered phase I clinical trials.
Type IV collagenase (also called gelatinases including MMP-2 and -9), as the main member of MMPs family, is of particular interest in the study of antibody-based drugs because the enzyme plays an important role in cancer invasion and metastasis. Using type IV collagenase as a molecular target, mAb 3G11 directed against MMP-2 and -9 was herein produced, analyzed, and evaluated for targeting cancer therapy. Previous studies have demonstrated that 3G11-LDM immunoconjugate remarkably suppresses the growth of hepatoma 22 (H22) and increases the survival time of tumor bearing mice. Moreover, the antitumor efficacy of the conjugate was higher than that of free LDM or mAb 3G11 alone[6]. In order to reduce the molecular size of agent, e.g. from the mAb to Fab and even to the Fv, a series of antibody-based drugs have been studied in our laboratory[7,8]. Utilizing anti-tumor and anti-metastasis mAb 3G11 as a therapeutic agent or as a carrier in cancer treatment may enhance the selectivity of chemotherapy.
In this study, we observed the immunoreacitivity of mAb 3G11 with 32 cases of human colorectal carcinoma specimens by IHC staining. For further characterization of the mAb, Western blot assay, ELISA, and gelatin zymography were performed. mAb 3G11 was used as a targeting carrier for colon cancer as it demonstrated a highly specific immunoreactivity to human colorectal carcinoma. 3G11-LDM immunoconjugate was prepared and its antitumor efficacy against colon carcinoma was examined.
Highly purified LDM was prepared in our institute. MTT was obtained from Sigma Chemical Co., (St. Louis, MO, USA). Mitomycin C (MMC) was purchased from Kyowa Hakko Kogyo Co., Ltd.
mAb 3G11, a murine IgG1-type mAb, was prepared using type IV collagenase (SIGMA Inc.) to immunize BALb/c mice. According to our previous methods, mAb 3G11 was purified by sequential affinity and size-exclusion chromatography on protein G and Superose-12 columns and then used for conjugation with LDM immediately. The 3G11-LDM conjugate was formed by heterobifunctional crosslinking reagent m-maleimidobenzoyl-N-hydroxy-succinimide ester (MBS) through linkage of the amino group of LDM apoprotein with the 3G11 molecule at molecular ratio of 1:1 in our laboratory.
The following cancer cell lines were used: human colon carcinoma HT-29 cells, human fibrosarcoma HT-1080 cells, human breast carcinoma MCF7 cells, mouse colon carcinoma 26 cells, mouse hepatoma 22 cells. All the cell lines were cultured in RPMI 1640 medium (Gibco BRL Inc.) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco BRL Inc.), 0.03% L-glutamine, 100 μg/mL streptomycin and 100 IU/mL penicillin at 37 °C in a humidified atmosphere containing 50 mL/L CO2. For use in experiments, cells grown in exponential phase were disaggregated to single cells by treatment with trypsin/EDTA for 2 min.
Tumor tissue specimens collected by standard surgical oncology procedures were obtained from the Pathology 20% glycerin, 2% SDS, 0.1% bromophenol blue. Samples (30 μL) were then separated by electrophoresis on 10% polyacrylamide gel containing 0.1% SDS and 1% gelatin as a substrate. Thereafter, gels were washed in the reaction buffer (50 mmol/L Tris-HCl, pH 7.6, 0.15 mol/L NaCl, 10 mmol/L CaCl2, 0.02% NaN3) containing 2.5% Triton-X 100 for 1 h to remove SDS. During this process, pro-gelatinases A and B were autocatalytically activated in situ. Gels were then incubated for 24 h at 37 °C in the reaction buffer and stained with 0.1% Coomassie-brilliant blue R-250. The location of gelatinolytic activity was detectable as a clear band in the background of uniform staining. The blank bands were scanned and saved by the image system (AIO Inc.).
Above-mentioned single-cell suspension volume was placed into the poly-L-lysine-coated 96-well ELISA plates (Costar Inc.) containing 1105 cells/100 μL per well at 4 °C overnight. After being blocked with a solution of BSA (1 mg/100 mL in PBS) and washed thrice with 0.05% Tween-20 in PBS (PBST), plates were incubated with primary antibody (mAb 3G11 or 3G11-LDM conjugate) and goat-anti-mouse IgG-conjugated horseradish peroxidase for 1 h at 37 °C, respectively. Following six washes with PBST, o-phenylenediamine-hydrogen peroxide (OPD-H2O2) substrate was used as a chromogen for visualization. Finally, the reaction was stopped by the addition of 100 μL of 0.1 mol/L H2SO4, and the absorbance readings (490 nm) were taken using a microplate reader (Bio-Rad Inc.).
Cells were detached by trypsinization and seeded at 3 000 cells/well in a 96-well plate (Costar, Cambridge, MA, USA) overnight. Then different concentrations of LDM, adriamycin (ADM), and MMC were added and incubated for an additional 48 h. The effect on cell growth was examined by MTT assay. Briefly, 20 μL of MTT solution (5 mg/mL) was added to each well and incubated at 37 °C for 4 h. The supernatant was aspirated, and the MTT formazan formed by metabolically viable cells was dissolved in 150 μL of DMSO, and then monitored by a microplate reader (Bio-Rad) at a wavelength of 560 nm.
Cancer cells at the concentration of 50 cells/well were seeded in 96-well plates and cultured for 24 h, then treated with LDM or 3G1-LDM at 37 °C for 1 h. Subsequently they were incubated for 5-7 d. Colonies formed were scored microscopically and the survival fractions (% control) were calculated using the following formula: survival fraction = (colony number of control well-colony number of treated well)/colony number of control well.
Female BALB/c mice (20±2 g, 7 wk of age, obtained from the Institute for Experimental Animals, CAMS) were inoculated subcutaneously with murine colon carcinoma 26 cells (1.5106 cells/mouse). Mice were divided randomly into untreated control group and six treatment groups, and carefully monitored for general well-being, body weight, and tumor size. Diameter of the tumor was measured twice a week with a caliper. Tumor volume was calculated with the following formula: v = 1/2ab2, where a and b are the long diameter and its perpendicular short diameter of the tumor, respectively. The data were presented as mean±SD. Student’s t-test was used to determine statistically significant differences.
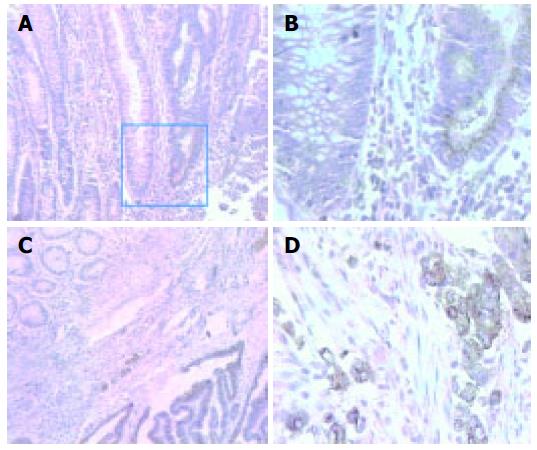
MAb 3G11 showed positive immunoreactivity in 81.3% (26/32) of cases of colorectal carcinoma, 43.8% (7/16) of cases of mammary carcinoma, 63.6% (7/11) of cases of gastric carcinoma, and 66.7% (4/6) of cases of esophageal carcinoma, respectively. Cytoplasmic staining tended to be strongest in the cells at the periphery or invasive margin of the tumors and in the cells at the margin of tumor nests, whereas adjacent non-neoplastic tissues showed negative staining (Figure 1).
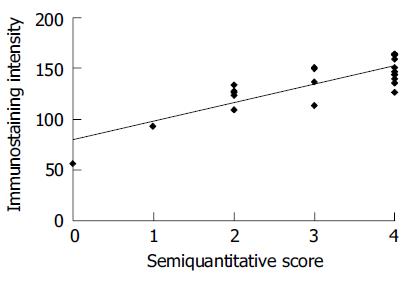
Moreover, we found a significant correlation between the evaluation method of 3G11 immunostaining by computer image analysis and by semi-quantitative scoring (Figure 2). The consistent results assessed by both manual methods and quantitative image analysis system proved that 3G11 showed highly specific immunoreactivity with human colon carcinomas and that quantitaive estimation might also serve as an useful approach for IHC analysis.
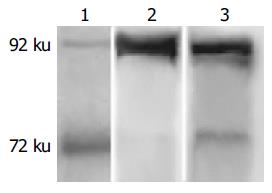
MAb 3G11 was characterized by Western blot analysis and two specific electrophoretic bands of 72 and 92 ku in the cell or tissue lysates are shown, supporting the IHC results (Figure 3). The appearance of two bands indicated that other matrix metalloproteinase present in the samples did not cross-react with mAb 3G11. The intensity of two bands of 72 and 92 ku was different in various cell lines and tissue lysates. The results indicated that the expression level of gelatinase was different in various tumors.
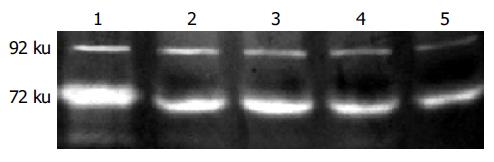
Both active and latent species could be visualized by using this technique. It showed that the clearance of the gelatin substrate by gelatinases with 72 and 92 ku was detected as a negative staining band, respectively. As shown, both the secreted activity of 72 and 92 ku gelatinases in human colon carcinoma HT-29 cells was inhibited by mAb 3G11 in a dose-dependent manner (Figure 4).
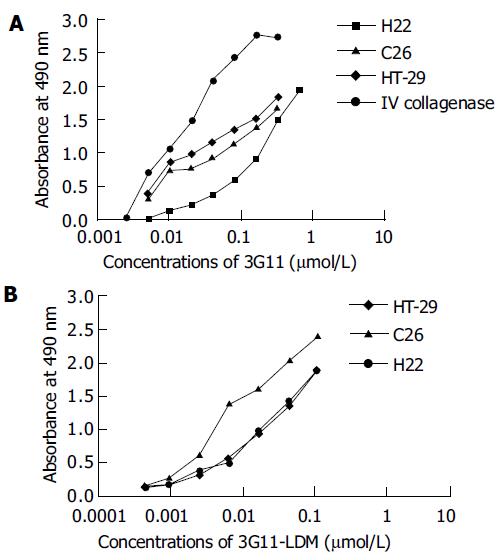
The binding of mAb 3G11 to antigen-related cancer cells including HT-29, H22, and C26 cells, which expressed type IV collagenases including MMP-2 and -9, is shown in a concentration-dependent manner ranging from 0.01 to 0.3 μmol/L of 3G11 (Figure 5A). The immunoconjugate 3G11-LDM still retained the immunoreactivity at a range of concentrations between 0.01 and 0.1 μmol/L (Figure 5B).
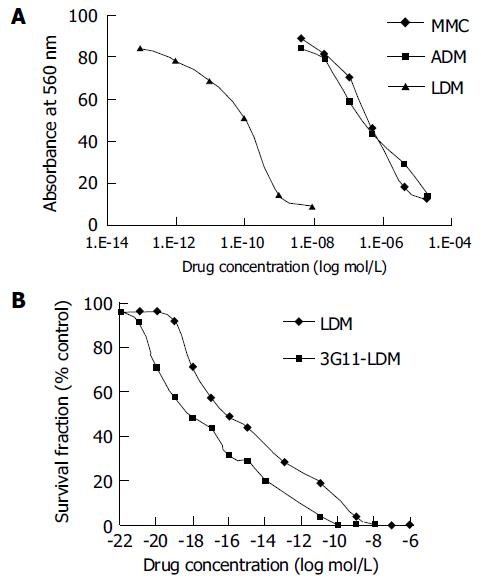
As determined by MMT assay, the IC50 values for LDM, ADM, and MMC to human colon carcinoma HT-29 cells were 0.0420.007, 528.397.7, and 992.093.0 nmol/L, respectively (Figure 6A).
In terms of IC50 values, the effect of LDM on HT-29 cells was 10 000-fold more potent than that of ADM and MMC.
By clonogenic assay, the conjugate 3G11-LDM displayed extremely potent cytotoxicity to human colon carcinoma HT-29 cells (Figure 6A). The IC50 value of 3G11-LDM for HT-29 cells was 5.6×10-10 nmol/L and that of the free LDM was 3.48×10-9 nmol/L.
According to the results on d 10 (Table 1), the tumor growth of colon carcinoma 26 in mice treated with 3G11-LDM was significantly inhibited by 54.5%, 70.3%, and 81.2% at the dose of 0.025, 0.05, and 0.1 mg/kg, respectively. It proved that 3G11-LDM showed higher anti-tumor efficacy against colon carcinoma in mice, as compared to the results of either free LDM or mAb 3G11.
| Groups | Doses (mg/kg) | Mice number (begin/end) | BWC (g) | Tumor volume (cm3) mean±SD | Inhibition rate (%) |
| Control | 10/10 | 0.5 | 1.0±0.20 | ||
| 3G11 | 0.75 | 10/10 | 0.6 | 0.8±0.20 | 25.7b |
| LDM | 0.05 | 10/10 | -0.2 | 0.4±0.16 | 56.4b |
| 3G11+ | 0.75 | 10/10 | -0.3 | 0.7±0.13 | 38.6b |
| LDM | +0.05 | ||||
| 3G11-LDM | 0.025 | 10/10 | -0.4 | 0.5±0.09 | 54.5ab |
| 3G11-LDM | 0.05 | 10/10 | -0.3 | 0.3±0.06 | 70.3ab |
| 3G11-LDM | 0.1 | 10/10 | -0.3 | 0.2±0.06 | 81.2ab |
Though numerous patients with alimentary tract carcinoma could be successfully induced into first remission by chemotherapy or radiotherapy, most of these patients ultimately have a relapse within 3-5 years. In addition, chemotherapy is accompanied with substantial side effects because of the non-specificity of the cytotoxic drugs. Therefore, it is clear that novel or additional therapy approaches are required. A promising therapy is antibody-targeted chemotherapy, which allows the specific delivery of cytotoxic drugs to the tumor tissues and is likely to have minimal toxic side effects[11,12]. Owing to its high potency cytotoxicity and molecular constitution, LDM may be successfully applied as a promising “warhead” in the production of antibody-based targeting therapeutics. The FDA-approved Zevalin and Bexxar are radioimmunotherapeutics derived from murine mAb. Their clinical application indicates that murine mAb may be used for cancer therapy under certain circumstances.
MMPs are now known to contribute tumor progression in addition to invasion, including tumor promotion, angiogenesis, and the establishment and growth of metastatic lesions in distant organ sites[13]. There is evidence that the expression of MMP-2 and -9, two of the most important MMPs, are linked to enhanced tumor angioge-nesis, tumor invasion, and metastasis[14-16]. By IHC staining in our studies, mAb 3G11 showed significant immunoreactivity with gelatinases in various human tumor tissues, especially in human colon carcinoma specimens, and no cross-reaction with adjacent non-neoplastic tissues was found. The IHC staining of mAb 3G11 was found to localize in most colon carcinoma cells and a few of surrounding fibroblasts. Our IHC results are in accordance with some previous reports[17]. MMP-2 and MMP-9 expression is more frequently found at the lateral and deep margins of the tumors as observed by immunohistological staining. Among the examined human cancer specimens, mAb 3G11 had the strongest staining intensity and the highest positive rate in colon carcinomas, suggesting that mAb 3G11 has highly specific immunoreactivity with human colon carcinoma and this is the key reason for the use of mAb 3G11 as a carrier for antibody-based therapy.
Inhibitors of MMPs (MMPIs) can be used to halt the spread of cancer. MMPIs do not directly kill cancer cells, but instead targets processes such as cancer cell invasion and metastasis. However, many initial clinical trials using MMPIs proved to be disappointing[18]. 3G11-LDM is a conjugate composed of a mAb directed against type IV collagenase, including MMP-2 and -9, and LDM displays extremely potent cytotoxicity. This antibody can inhibit the enzyme activity and selectively bind to the target enzyme in tumor tissues. 3G11-LDM conjugate has potent antitumor efficacy both in vivo and in vitro.
In conclusion, 3G11-LDM is a promising agent for targeted cancer chemotherapy, especially for colorectal carcinoma.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Xu YJ, Zhen YS, Goldberg IH. C1027 chromophore, a potent new enediyne antitumor antibiotic, induces sequence-specific double-strand DNA cleavage. Biochemistry. 1994;33:5947-5954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Shao RG, Zhen YS. Relationship between the molecular composition of C-1027, a new macromolecular antibiotic with enediyne chromophore, and its antitumor activity. Yaoxue Xuebao. 1995;30:336-342. |
| 3. | Wang XH, Wu SY, Zhen YS. Lidamycin inhibits prolification and induces apoptosis in endothelial cells. Zhongguo Kangshengsu Zazhi. 2003;28:605-612. |
| 4. | He QY, Liang YY, Wang DS, Li DD. Characteristics of mitotic cell death induced by enediyne antibiotic lidamycin in human epithelial tumor cells. Int J Oncol. 2002;20:261-266. [PubMed] |
| 5. | Liu W, Christenson SD, Standage S, Shen B. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science. 2002;297:1170-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Wang FQ, Shang BY, Zhen YS. Antitumor effects of the immunoconjugate composed of lidamycin and monoclonal antibody 3G11. YaoXue XueBao. 2003;38:515-519. [PubMed] |
| 7. | Liu XY, Zhen YS. Antitumor effect of lidamycin-containing monoclonal antibody immunoconjugate with downsized-molecule. Zhongguo YiXue KeXueYuan XueBao. 2001;23:563-567. [PubMed] |
| 8. | Li L, Huang YH, Miao QF, Shang BY, Zhen YS. Fv-LDP-AE, an engineered and enedyine-energized fusion protein, shows highly potent antitumor efficacy and antiangiogenic activity. Proc Am Assoc Cancer Res. 2004;45:2884. |
| 9. | Lehr HA, Jacobs TW, Yaziji H, Schnitt SJ, Gown AM. Quantitative evaluation of HER-2/neu status in breast cancer by fluorescence in situ hybridization and by immunohistochemistry with image analysis. Am J Clin Pathol. 2001;115:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Brown PD, Levy AT, Margulies IM, Liotta LA, Stetler-Stevenson WG. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990;50:6184-6191. [PubMed] |
| 11. | Zhen YS. Advances in research of antitumor antibiotics and monoclonal antibody therapeutics. Zhongguo Kangshengsu Zazhi. 2002;27:1-5. |
| 12. | Dickman S. Antibodies stage a comeback in cancer treatment. Science. 1998;280:1196-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 14. | Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2074] [Cited by in RCA: 2088] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 15. | Höyhtyä M, Fridman R, Komarek D, Porter-Jordan K, Stetler-Stevenson WG, Liotta LA, Liang CM. Immunohistochemical localization of matrix metalloproteinase 2 and its specific inhibitor TIMP-2 in neoplastic tissues with monoclonal antibodies. Int J Cancer. 1994;56:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Jeziorska M, Haboubi NY, Schofield PF, Ogata Y, Nagase H, Woolley DE. Distribution of gelatinase B (MMP-9) and type IV collagen in colorectal carcinoma. Int J Colorectal Dis. 1994;9:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bodey B, Bodey B, Siegel SE, Kaiser HE. Prognostic significance of matrix metalloproteinase expression in colorectal carcinomas. In Vivo. 2000;14:659-666. [PubMed] |
| 18. | Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res., 9: 00-00, 2003. Clin Cancer Res. 2003;9:551-554. [PubMed] |