Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4451
Revised: November 15, 2004
Accepted: November 19, 2004
Published online: August 7, 2005
AIM: To study the role of P38 kinase in esophageal cancer cell apoptosis induced by genotoxin, cisplatin and the unfolded protein response (UPR) inducer, dithiothreitol (DTT).
METHODS: Esophageal carcinoma cell line Eca109 was cultured in RPMI 1640 medium to 70% confluency and treated with either cisplatin, DTT, or cisplatin plus DTT in the presence or absence of P38 inhibitor, SB203580. The untreated cells served as the control. The esophageal carcinoma cell apoptosis was detected by agarose gel DNA ladder analysis and quantified by flow cytometry. The P38 phosphorylation was detected by immunohis-tochemistry using antibodies specific to phosphorylated P38 protein.
RESULTS: (1) Both cisplatin and DTT induced apoptosis in the esophageal cancer cell line Eca109 as shown by DNA ladder formation; (2) As detected by antibodies specific for the phosphorylated P38 protein (p-P38), both cisplatin and DTT treatments activated the stress-activated enzyme, MAP kinase P38. The number of positive cells was about 50% for the treatment groups, comparing to that of 10% for untreated group. DTT treatment, but not cisplatin treatment, induces nuclear localization of p-P38; (3) As measured by flow cytometry, inhibition of P38 activity by SB203580 blocks DTT- and cisplatin-induced apoptosis. The rates for DTT, cisplatin, and DTT plus cisplatin-induced apoptosis were 16.8%, 17.1%, and 21.4%, respectively. Addition of the SB compound during the incubation reduced the apoptotic rate to about 7.6% for all the treatment groups, suggesting that P38 activation is essential for cisplatin- and DTT-induced apoptosis in Eca109 cells.
CONCLUSION: (1) Both DTT and cisplatin were able to induce apoptosis in esophageal cancer cell line Eca109; (2) P38 MAP kinase is essential for DTT- and cisplatin-induced apoptosis in Eca109 cells; (3) P38 activation may be the common signaling component relaying the multiple upstream signaling events to the downstream cell death program.
- Citation: Zhang QX, Feng R, Zhang W, Ding Y, Yang JY, Liu GH. Role of stress-activated MAP kinase P38 in cisplatin- and DTT-induced apoptosis of the esophageal carcinoma cell line Eca109. World J Gastroenterol 2005; 11(29): 4451-4456
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4451.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4451
Apoptosis is a programmed cell death stringently controlled by cell signaling pathways and the expression of pro- and anti-apoptotic genes. Inactivation of pro-apoptotic genes or signaling pathways or activation of anti-apoptotic genes or signaling pathways would compromise the ability of the cell to undergo apoptosis, thus contributing to the genesis and development of cancer. Therefore, a thorough understanding of the signaling events leading to the apoptotic program in tumor cells may assist in the development of novel strategies for cancer therapy.
Mitogen-activated protein kinases (MAPK) are members of a family of serine/threonine protein kinases activated by dual phosphorylation at threonine 188 and tyrosine 190 positions. They mainly consist of ERK, JNK/SAPK, and P38. Despite structural similarities, MAPKs play diverse roles in regulating cell function. The activation of ERK promotes cell proliferation and survival, while activation of the stress-activated protein kinases JNK/P38 can mediate different cell responses ranging from stress-induced cell apoptosis to various inflammatory responses[1-5]. In different cells, the activation of P38 kinase is reportedly either pro-apoptotic or anti-apoptotic[6-10], and is likely to be determined by the type of stress stimuli and/or the state of cell proliferation and differentiation. Cisplatin is a genotoxin that causes nuclear damage and nuclear stress. It was found that JNK/P38 MAP kinase is activated during cisplatin-induced apoptosis in human lung and ovarian cancer cells[11,12], which might play an important role in signaling cancer cell apoptosis during cisplatin therapy. The unfolded protein response (UPR) or ER stress response is an ER-based cell stress response[13-15], which can be triggered by many agents such as DTT, tunicamycin, and thapsigargin[16-20]. These agents induce the UPR by interfering with protein folding in the endoplasmic reticulum (ER), thus resulting in the build up of unfolded proteins in the ER. Activation of the UPR triggers cellular compensatory responses such as slowing down of protein translation and speeding up the production of molecular chaperone and, when the rescue efforts fail, it induces cell apoptosis. Whether the activation of P38 is required for the UPR-mediated cell apoptosis remains to be elucidated.
Esophageal carcinoma is one of the most common and debilitating malignancies in China. It was reported that cisplatin could induce apoptosis in esophageal cancer cells[21]. However, the role of P38 MAP kinase in cisplatin-induced esophageal cancer cell apoptosis is unclear. In this study, it was tested whether P38 kinase is activated in cisplatin-treated esophageal cancer cells and, if it is, whether the P38 activation is an essential step for cisplatin-induced apoptosis. And it was further investigated whether P38 kinase activation is required for the UPR inducer DTT-induced apoptosis in esophageal cancer cells. This study may provide new insights into the role of P38 kinase in esophageal cancer cell apoptosis triggered by diverse cell stressors.
The anti-phosphorylated P38 antibodies were kindly provided by Dr. Tao Zhu, National University of Singapore. SB203580, DTT, RPMI 1640, and FBS were from Sigma. The other reagents were purchased from Beijing Zhongshan Biotechnology Co., Ltd.
Eca109 cells were provided by our department and grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin and 100 U/mL streptomycin. All cell cultures were done at 37 °C with 5 mL/L CO2 in a humidified incubator.
Eca109 cells were cultured to 70% confluency in RPMI 1640 medium. The cultures were continued in the RPMI 1640 medium for additional 24 h with or without 10 µg/mL cisplatin, 2 mmol/L DTT, or 10 µg/mL cisplatin plus 2 mmol/L DTT. For testing the effects of P38 inhibitor SB203580 on cell apoptosis, cells were pretreated with 10 µg/mL SB203580 for 2 h before switching to cisplatin, DTT or cisplatin+DTT containing media. Each experimental condition was set up in flask. The untreated cells were the control. At the end of the treatment, the cells were collected from the cell culture flasks and further analyzed either for cell apoptosis by DNA ladder formation, P38 activation by immunostaining or cell apoptosis by flow cytometry.
DNAs for fragmentation analysis were prepared as previously described[22]. DNA was analyzed for each condition by 1.5% agarose gel electrophoresis in TBE buffer. The gel was stained with ethidium bromide (0.5% µg/mL) and visualized under UV light.
Cells were harvested from the flasks, washed with cold PBS and fixed onto glass slides by using 40 g/L paraformaldehyde solution. p-P38 kinase was detected using anti-phosphorylated P38 antibody (1:200 dilution), according to the protocol of Beijing Zhongshan Biotechnology Co., Ltd. Negative control was provided by the untreated Eca109 cells, without the incubation with anti-phosphorylated P38 antibody. DAB staining system was used to demonstrate the amount of p-P38, which is the activated form of P38. The slides were counter-stained by Methyl Green dye to visualize the cell structure. The 100 cells from each condition were scored based on the immunostaining signal intensity as follows: -: 0, -/+: 0.5, +: 1, ++: 2.
The cells were harvested and washed twice with PBS (pH 7.2) and suspended in 80% ethanol at 20 °C for 24 h, then thawed quickly at room temperature and centrifuged to collect the cells. The cells were washed with PBS twice again and resuspended in the extraction buffer (0.2 mol/L Na2HPO4 0.1 mol/L citric acid) for 5 min, and finally re-suspended in PBS containing RNAse A (100 µg/mL) and 50 µg/mL propidium iodide for 30 min. The cell cycle distribution and quantitation of apoptotic cells were determined by the fluorescence of individual cells measured by flow cytometry (Beckman-Coulter Epics Altra).
Biostatistical analyses were done using SPSS10.0 software package. The data from immunohistochemistry and flow cytometry were analyzed by χ2 and Kruskal-Wallis tests.
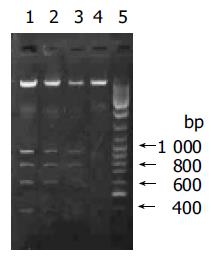
Cisplatin is known to induce cell death via apoptosis in many cell types, by damaging DNA and disturbing nuclear function. DTT, on the other hand, is a well-known ER stress inducer and induces cell apoptosis via UPR. A biochemical hallmark of apoptosis is the characteristic degradation of the genomic DNA by cleavage at the internucleosomal sites, generating a ‘ladder’ of DNA fragments, which can be detected by agarose gel electrophoresis[23-25]. To determine whether cisplatin and DTT induce Eca109 cell apoptosis, Eca109 cells were treated with cisplatin, DTT and cisplatin plus DTT. The total DNA was resolved on agarose gel. DNA ladders were observed for all three treatment groups, while only large molecular weight DNA with no obvious ladder formation was seen for the untreated cells (Figure 1). This result demonstrates that both cisplatin and DTT were able to induce apoptosis in esophageal cancer Eca109 cells. In addition, spontaneous apoptosis of Eca109 cells is minimal under these experimental conditions.
The DNA samples were analyzed by 1.5% agarose gel in TBE buffer and visualized by UV-ethidium bromide method. Lane 1: cisplatin plus DTT; lane 2: DTT; lane 3: cisplatin; lane 4: untreated cells; lane 5: DNA molecular weight marker. The DNA bands of 400, 600, 800, and 1 000 bp were clearly seen on lanes 1-3.
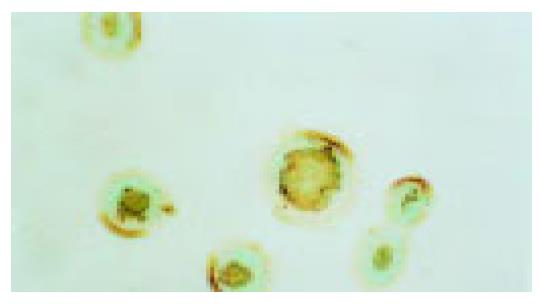
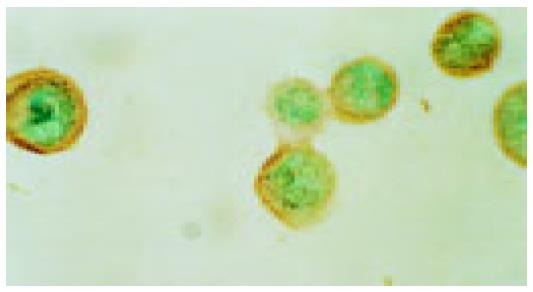
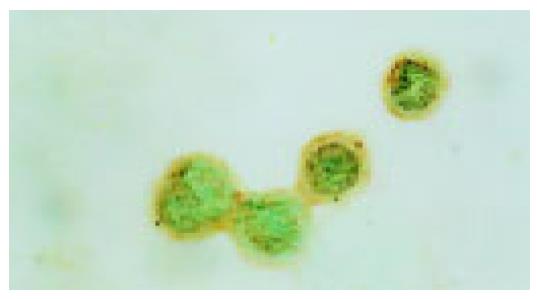
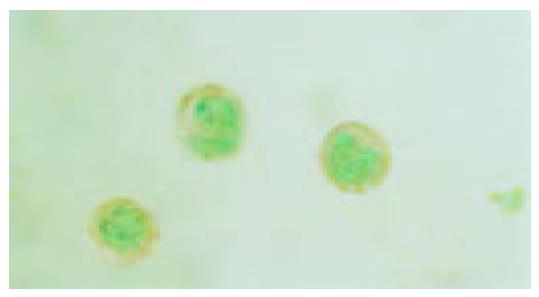
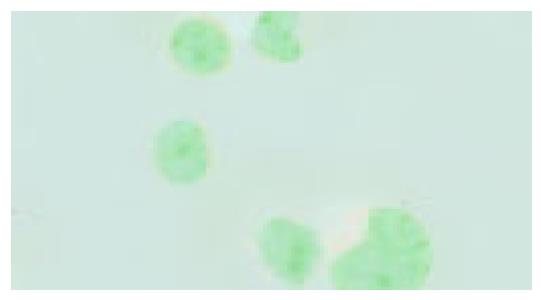
Activation of P38 kinase is achieved via the phosphorylation of P38 protein by the upstream kinases, which can be detected by antibodies specifically for the phosphorylated P38 protein (p-P38). To detect the P38 activation upon cell stresses, the Eca109 cells were cultured and treated with cisplatin, DTT, and cisplatin plus DTT for 24 h. The cells were stained with anti-p-P38 antibodies. The untreated Eca109 cells without incubation with anti-p-P38 antibodies were used as negative control. The cells were grouped into four groups, based on their signal intensity for the immunostaining. The percentage of cells positively stained with anti-p-P38 antibodies are summarized in Table 1.
Both cisplatin and DTT treatments markedly increased the number of the cells positive for p-P38 kinase. About 50% of the treated cells were positive for p-P38, when compared to that of 10% for the untreated cells. Few positive cells were observed in negative control. Moreover, comparing with the untreated group, the level of the phosphorylated P38 was dramatically increased upon treatment with cisplatin and DTT (P<0.01). Treatment with DTT in combination with cisplatin showed only a modest increase in the number of the positive cells and the level of p-P38 signal. The difference did not reach statistical significance (P<0.05).
Interestingly, the subcellular localization of p-P38 kinase was markedly different between DTT, and cisplatin-treated cells (Figure 2, Figure 3, Figure 4, Figure 5) though both treatments achieved the same level of P38 activation. The p-P38 was largely localized in the nucleus for DTT-treated cells, while the distribution of p-P38 for cisplatin-treated cells was mostly cytoplasmic, accentuated in the peri-plasma membrane region. Addition of DTT to the cisplatin treatment resulted in partial nuclear localization of the p-P38 kinase. The significance of the different localization of p-P38 by different death inducers is unclear.
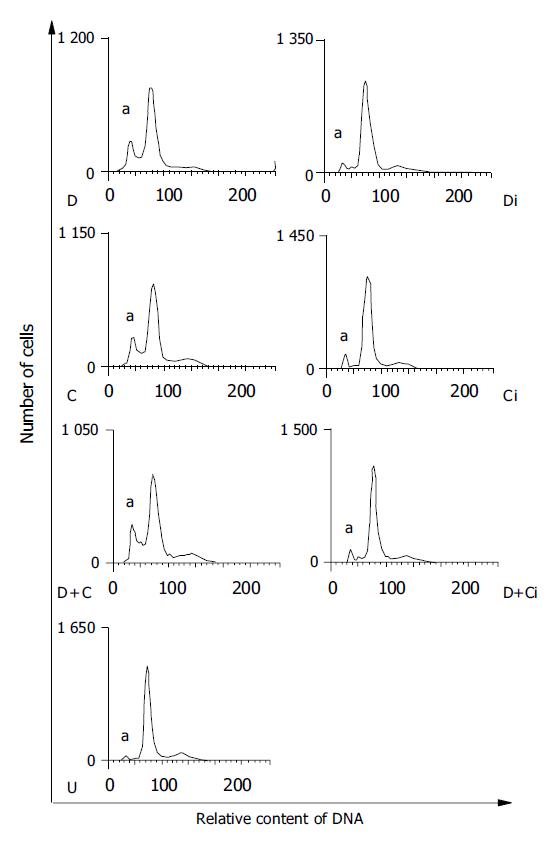
To test whether the activation of P38 kinase is required for DTT- and cisplatin-induced Eca109 cell apoptosis, the P38 kinase inhibitor SB203580 was employed to inhibit P38 kinase activity in DTT- and cisplatin-treated cells. The rate of apoptosis was determined by flow cytometry analysis. The data are shown in Figure 6 and summarized in Table 2. The rates of Eca109 cell apoptosis were 16.8%, 17.1%, and 21.4% for DTT, cisplatin, and DTT plus cisplatin, respectively. Consistent with the data of P38 kinase activation, DTT and cisplatin induced apoptosis to a similar extent. The rate of apoptosis for DTT plus cisplatin-treated cells was moderately higher. Remarkably, SB203580 compound effectively inhibited Eca109 cell apoptosis in all three treatment groups (P<0.01). These results strongly suggest that P38 activation is essential for both cisplatin- and DTT-induced apoptosis in Eca109 cells. The fact that both DTT- and cisplatin-induced cell apoptosis requires P38 activity implies that P38 kinase might be the common component shared by both stress response pathways (Figure 7).
As a commonly used chemotherapeutic agent, cisplatin exerts its cytotoxic effect by intrastrand and interstrand crosslinking of DNA at specific base sequences[26]. Recent studies have shown that the mechanism of cisplatin-induced cell death is through apoptosis[21]. It was found that P38 MAP kinase is activated during cisplatin-induced apoptosis in human lung and ovarian cancer cells, which might play an important role in signaling cancer cell apoptosis during cisplatin therapy. However, no reports showed the role of P38 MAP kinase in cisplatin-induced esophageal cancer cell apoptosis. Our studies show that cisplatin treatment strongly activates P38 kinase in esophageal cancer cells. Moreover, inhibition of P38 activity by the specific inhibitor SB203580 almost completely blocked cisplatin-induced apoptosis. Our results and the data reported by others[11,12] support the notion that P38 activation is a critical step in cisplatin-induced apoptosis.
The exact position of P38 activation in the chain of signaling pathways leading to the execution of cell death program is still undetermined. Previous data suggest that P38 kinase is involved in histone H3 phosphorylation[27], nuclear condensation, and cell blebbing[28] during cisplatin-induced apoptosis. It is conceivable that the activation of P38 kinase is an early intermediate event branching out from the cell stress response pathways but before the execution of cell death program.
DTT is known to induce the unfolded protein response, probably by perturbing the ER oxidoreductive potential, important for disulfide bond formation and protein folding. Several branches of the UPR can lead to cell death program[29]. Few studies focus on the effects of DTT on apoptosis and none of the reports had showed the relationship between UPR and P38 MAP kinase signaling pathway. Our data indicate that DTT treatment effectively triggered Eca109 cell apoptosis with potency comparable to that of cisplatin. Remarkably, P38 kinase was strongly activated by DTT treatment. This provides the first link between the UPR and P38 kinase activation. Furthermore, inhibition of P38 kinase activity blocks DTT-induced apoptosis, indicating that P38 activation is an indispensable intermediate step for the UPR-mediated cell apoptosis. It was reported previously that Japanese encephalitis virus (JEV) infection triggers the UPR and apoptosis in fibroblast and neuronal cells[30]. Japanese encephalitis virus-induced apoptosis can be partially blocked by P38 kinase inhibitor SB203580.
An intriguing finding was that the subcellular localization of the activated P38 was markedly different in DTT- and cisplatin-treated cells. In DTT-treated cells, p-P38 was mainly seen in the nucleus, while in cisplatin-treated cells, p-P38 is mostly localized in the cytoplasm. The basis for this observation remains speculative. One possibility is that the nuclear perturbation exerted by cisplatin treatment hinders the p-P38 nuclear entry or retention. Surprisingly, the different localization of p-P38 does not significantly affect the apoptotic rate of the treated cells. This might suggest that p-P38 localization is not essential for p-P38 to relay its signal to the downstream apoptotic pathways. Alternatively, the nuclear fraction and cytoplasmic fraction of the p-P38 might trigger different downstream signaling pathways, which are able to transduce the signal to cell death program with similar efficacies. More studies are needed to address this issue.
The data obtained here raises the possibility that P38 kinase is the common central component relaying multiple cell stress pathways to the cell death program. If this notion proved to be true, then it might be possible to develop anticancer agents, which could specifically activate P38 kinase along with minimal nonspecific toxicities. This might also help to formulate newer drug combinations with synergistic activation of P38 kinase, to achieve better therapeutic efficacy and lesser side effects. Furthermore, DTT might be another apoptosis inducer. More studies should be carried out using different cell lines.
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
| 1. | Kim BS, Yoon KH, Oh HM, Choi EY, Kim SW, Han WC, Kim EA, Choi SC, Kim TH, Yun KJ. Involvement of p38 MAP kinase during iron chelator-mediated apoptotic cell death. Cell Immunol. 2002;220:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Petrich BG, Wang Y. Stress-activated map kinases in cardiac remodeling and heart failure; new insights from transgenic studies. Trends Cardiovasc Med. 2004;14:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Hashimoto K, Farrow BJ, Evers BM. Activation and role of MAP kinases in 15d-PGJ2-induced apoptosis in the human pancreatic cancer cell line MIA PaCa-2. Pancreas. 2004;28:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Scali C, Giovannini MG, Prosperi C, Bellucci A, Pepeu G, Casamenti F. The selective cyclooxygenase-2 inhibitor rofecoxib suppresses brain inflammation and protects cholinergic neurons from excitotoxic degeneration in vivo. Neuroscience. 2003;117:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Choi JA, Park MT, Kang CM, Um HD, Bae S, Lee KH, Kim TH, Kim JH, Cho CK, Lee YS. Opposite effects of Ha-Ras and Ki-Ras on radiation-induced apoptosis via differential activation of PI3K/Akt and Rac/p38 mitogen-activated protein kinase signaling pathways. Oncogene. 2004;23:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Schrantz N, Bourgeade MF, Mouhamad S, Leca G, Sharma S, Vazquez A. p38-mediated regulation of an Fas-associated death domain protein-independent pathway leading to caspase-8 activation during TGFbeta-induced apoptosis in human Burkitt lymphoma B cells BL41. Mol Biol Cell. 2001;12:3139-3151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000;60:2464-2472. [PubMed] |
| 8. | Liu HR, Tao L, Gao E, Lopez BL, Christopher TA, Willette RN, Ohlstein EH, Yue TL, Ma XL. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc Res. 2004;62:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Lee RJ, Albanese C, Stenger RJ, Watanabe G, Inghirami G, Haines GK, Webster M, Muller WJ, Brugge JS, Davis RJ. pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. J Biol Chem. 1999;274:7341-7350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Viktorsson K, Ekedahl J, Lindebro MC, Lewensohn R, Zhivotovsky B, Linder S, Shoshan MC. Defective stress kinase and Bak activation in response to ionizing radiation but not cisplatin in a non-small cell lung carcinoma cell line. Exp Cell Res. 2003;289:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, Siddik ZH, Mills GB, Claret FX. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem. 2003;278:19245-19256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 740] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 14. | Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1104] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 15. | Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935-25938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 462] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Gilmore WJ, Kirby GM. Endoplasmic reticulum stress due to altered cellular redox status positively regulates murine hepatic CYP2A5 expression. J Pharmacol Exp Ther. 2004;308:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Trotter EW, Grant CM. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol Microbiol. 2002;46:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Martínez IM, Chrispeels MJ. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell. 2003;15:561-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 323] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Piccini A, Fassio A, Pasqualetto E, Vitali A, Borghi R, Palmieri D, Nacmias B, Sorbi S, Sitia R, Tabaton M. Fibroblasts from FAD-linked presenilin 1 mutations display a normal unfolded protein response but overproduce Abeta42 in response to tunicamycin. Neurobiol Dis. 2004;15:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Mulder HJ, Saloheimo M, Penttilä M, Madrid SM. The transcription factor HACA mediates the unfolded protein response in Aspergillus niger, and up-regulates its own transcription. Mol Genet Genomics. 2004;271:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Raouf A, Evoy D, Carton E, Mulligan E, Griffin M, Sweeney E, Reynolds JV. Spontaneous and inducible apoptosis in oesophageal adenocarcinoma. Br J Cancer. 2001;85:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Nasreen N, Mohammed KA, Dowling PA, Ward MJ, Galffy G, Antony VB. Talc induces apoptosis in human malignant mesothelioma cells in vitro. Am J Respir Crit Care Med. 2000;161:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Kim JY, Kim KM, Nan JX, Zhao YZ, Park PH, Lee SJ, Sohn DH. Induction of apoptosis by tanshinone I via cytochrome c release in activated hepatic stellate cells. Pharmacol Toxicol. 2003;92:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Scavo LM, Newman V, Ertsey R, Chapin CJ, Kitterman JA. Maternally administered dexamethasone transiently increases apoptosis in lungs of fetal rats. Exp Lung Res. 2003;29:211-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Yeh CH, Wang YC, Wu YC, Chu JJ, Lin PJ. Continuous tepid blood cardioplegia can preserve coronary endothelium and ameliorate the occurrence of cardiomyocyte apoptosis. Chest. 2003;123:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Zamble DB, Mikata Y, Eng CH, Sandman KE, Lippard SJ. Testis-specific HMG-domain protein alters the responses of cells to cisplatin. J Inorg Biochem. 2002;91:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Wang D, Lippard SJ. Cisplatin-induced post-translational modification of histones H3 and H4. J Biol Chem. 2004;279:20622-20625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Deschesnes RG, Huot J, Valerie K, Landry J. Involvement of p38 in apoptosis-associated membrane blebbing and nuclear condensation. Mol Biol Cell. 2001;12:1569-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Kudo T. [Involvement of unfolded protein responses in neurodegeneration]. Nihon Shinkei Seishin Yakurigaku Zasshi. 2003;23:105-109. [PubMed] |
| 30. | Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol. 2002;76:4162-4171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |