Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4210
Revised: October 15, 2004
Accepted: October 18, 2004
Published online: July 21, 2005
AIM: Itopride is a newly developed prokinetic agent, which enhances gastric motility through both antidopaminergic and anti-acetylcholinesterasic actions. The importance of esophageal motor dysfunction in the pathogenesis of gastro-esophageal reflux disease (GERD) makes it interesting to examine the effect of itopride on esophageal acid exposure.
METHODS: The effect of itopride on esophageal acid reflux variables for 24 h was studied in 26 patients with GERD symptoms, pre-entry total acid exposure time (pH<4) of more than 5% and mild esophagitis (Savary-Miller grades I, II) proven by endoscopy. Ambulatory 24-h pH-metry and symptom assessment were performed after treatments with 150 or 300 mg itopride thrice a day (t.i. d.) for 30 d in random order, using an open label method. For evaluating the safety of itopride, blood biochemical laboratory test was performed and the serum prolactin level was also examined before and after treatment.
RESULTS: Total symptom score was significantly decreased after treatment in 150- or 300-mg group. Itopride 300 mg was significantly effective than 150 mg on decreasing the total per cent time with pH < 4, total time with pH < 4 and DeMeester score. No serious adverse effects were observed with administration of itopride in both groups.
CONCLUSION: Itopride 100 mg t.i.d. is effective on decreasing pathologic reflux in patient with GERD and therefore it has the potential to be effective in the treatment of this disease.
- Citation: Kim YS, Kim TH, Choi CS, Shon YW, Kim SW, Seo GS, Nah YH, Choi MG, Choi SC. Effect of itopride, a new prokinetic, in patients with mild GERD: A pilot study. World J Gastroenterol 2005; 11(27): 4210-4214
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4210
Gastro-esophageal reflux disease (GERD) is characterized by the reflux of gastric content into esophagus with or without histological changes. However, the excessive reflux of gastric acid induces some complications such as esophagitis, esophageal stenosis, cancer, or Barrett’s esophagus. Pathogenesis of GERD are lower esophageal sphincter (LES) dysfunction, abnormal clearing capacity of refluxed materials, delayed gastric emptying and abnormal resistance of esophageal mucosa to gastric acid, but the primary motor dysfunction is regarded as the most important factor in general[1,2]. Therefore, prokinetic agent that restores gastric motility with increasing of LES and esophageal motility has been developed and used frequently in the treatment of GERD[3]. There are several prokinetic agents such as bethanechol, metoclopramide, domperidone, and cisapride that potentiate acetylcholine effects on smooth muscle of gastrointestinal tract or antagonize the activity of dopamine.
However, these prokinetic agents have some limitations for prescribing to GERD patients, because they show uncertain efficacies on GERD except for cisapride and have some problems in safety[4,5]. Especially, cisapride accelerates the esophageal acid clearance by an enhancement of esophageal propulsive motility, which strengthens LES and stimulates the gastric emptying. So it is regarded that cisapride has the same efficacy as an inhibitor of gastric acid secretion for GERD. In spite of these predominant effects, cisapride is restricted to use because of a safety problem that it provokes arrhythmia.
Itopride, a new type of the prokinetic agent, acts both as a dopamine D2 receptor antagonist and as an acetylcholine esterase inhibitor. It accelerates gastric emptying, improves gastric tension and sensitivity, and has an anti-emetic action. And also, itopride was identified to have equivalent efficacy with cisapride in functional dyspepsia. These mechanisms of itopride may be useful to GERD patients, but no clinical data are available from them as yet.
The aim of this pilot study is to evaluate the efficacy of itopride for the treatment of GERD by evaluating the improvement of the clinical symptom and esophageal pH change.
This study was performed prospectively to evaluate the efficacy and dose-response of itopride for the treatment of GERD by estimating ambulatory 24-h pH-metry and clinical symptoms before and after treatment. Patients satisfying the entry criteria were assigned to two groups by the randomization allocation schedule and received either 150 or 300 mg of itopride per day.
Twenty-six patients (range 18-65 years) who visited the Department of Internal Medicine in Wonkwang University and agreed to participate in this study were recruited based on the following entry criteria: Inclusion criteria were (1) more than moderate heartburn symptom; (2) more than one upper dyspeptic symptoms such as regurgitation, epigastric pain, nausea, vomiting, dysphagia, chest pain lasting for more than 4 wk; (3) less than grade II esophagitis by Savary-Miller classification by endoscopic examination; (4) abnormal acid exposure on 24-h pH-metry (fraction of time of >5% with pH below 4).
For each symptom, the severity scores are as follows: 0 (none) = absent; 1 (mild) = symptom was not spontaneously reported but elicited upon specific questions; 2 (moderate) = symptom needs to be treated but not interfered with normal activities; 3 (severe) = symptom interfered with normal activities; 4 (very severe) = impossible to do normal activities due to symptom.
Exclusion criteria were the following: (1) acute esophagitis related to strong stimuli; (2) corrosive esophagitis by a toxicant; (3) esophagitis or some symptoms by inflammatory infection or radiotherapy; (4) regular use of H2 blockers, prokinetic or anticholinergic agents for previous 4 wk; (5) previous gastrointestinal surgery; (6) inflammatory bowel disease; (7) cardiological, respiratory, gastrointestinal disease, endocrine metabolic disease and neuro-psychological disease; (8) clinically significant hepatic or renal dysfunction; (9) pregnancy or lack of adequate contraception in the case of women at child-bearing age; (10) patients who are inappropriate to this study by investigator’s opinion.
This study was approved by the institutional review board of Wonkwang University Hospital and informed consent was obtained from patients for inclusion in this study. The patients were asked about basic information and previous treatment history. And they were screened as physical examination, vital sign and lab examination. Any drugs that could influence the study were postponed for at least 1 wk before that examination.
The baseline symptom of GERD was evaluated by questionnaire. Endoscopic examination was performed to check the organic lesion like cancer or ulcer, and to evaluate the severity of esophagitis. After examination, ambulatory 24-h pH-metry was carried out.
Patients who met the entry criteria were allocated randomly to 150- or 300-mg group of itopride. Fifty or 100 mg of itopride was administered thrice per day for 4 wk. After treatment of itopride for 4 wk, physical examination, vital sign, laboratory test, and symptoms of GERD were evaluated and ambulatory 24-h pH-metry was performed.
Efficacy parameters were (1) changes in acid reflux episodes measured by ambulatory pH-metry such as number of reflux episodes, number of reflux episodes lasting for more than 5 min, duration of longest episodes, % time with pH < 4, and DeMeester score; (2) changes in subjective symptom of patient with GERD.
Safety parameters were (1) results of laboratory test (biochemistry, complete blood count, urinalysis and prolactin level); (2) occurrences of adverse events after the treatment. If adverse events occurred, the time of onset, duration, severity, relationship to itopride and the requirement of treatment were evaluated.
Two sample t-test and paired t-test were used for continuous variables presented as a normal distribution; Wilcoxon signed rank test and Wilcoxon rank sum test were used for those not presented as a normal distribution. And χ2 test or Fisher’s exact test was used for categorical variables. All data were statistically analyzed with SAS package (version 8.0) and differences with a value of P < 0.05 were considered significant.
The mean age and body weight of patients in 150-mg group were 44 ± 9 years and 64 ± 11 kg, those of patients in 300-mg group were 45 ± 12 years and 61 ± 12 kg. Seven were men, six were women in 150-mg group and five were men, eight were women in 300-mg group.
Baseline characteristics of patients in two groups are detailed in Table 1 and no statistical differences were noted in the past history, smoking and drinking history and endoscopic results.
| 150 mg | 300 mg | P | ||
| Age (yr) | 44 ± 9 | 45 ± 12 | 0.8141 | |
| Sex | Male | 7 | 5 | 0.734 |
| Female | 6 | 8 | ||
| Weight | 64 ± 11 | 61 ± 12 | 0.4281 | |
| Duration of reflux disease | < 6 mo | 5 | 2 | 0.412 |
| 6 -2 yr | 6 | 8 | ||
| > 2 yr | 2 | 3 | ||
| Pre-medication | Yes | 2 | 1 | 0.828 |
| No | 11 | 12 | ||
| Endoscopy | 0 | 5 | 7 | 0.717 |
| (Savary-Miller grade) | ||||
| I | 7 | 5 | ||
| II | 1 | 1 | ||
| Drinking | Yes | 2 | 3 | 0.884 |
| No | 11 | 10 | ||
| Smoking | Yes | 3 | 3 | 1 |
| No | 10 | 10 | ||
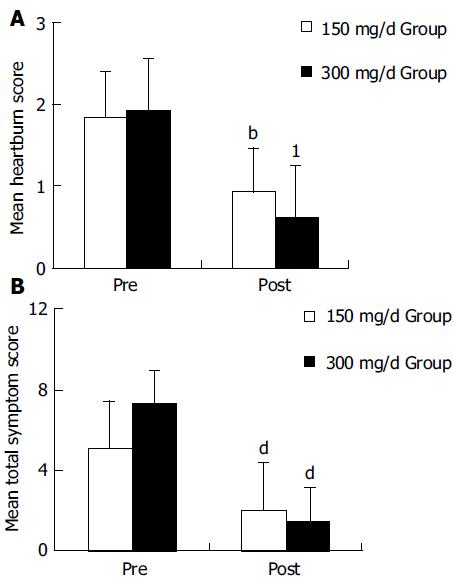
A significant improvement of all symptoms including heartburn, a main symptom of GERD, was identified after the 150 and 300 mg of itopride treatment compared with the pre-treatment (Figure 1A). No statistically significant differences were found between two groups in the symptom improvement (Figure 2B).
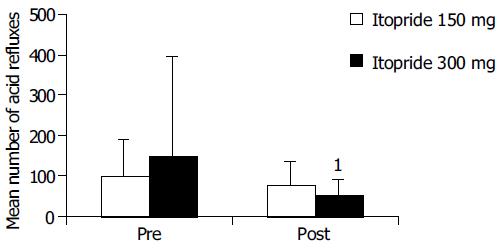
Among the acid reflux variables recorded, total time of pH < 4, per cent time with pH<4 and DeMeester score were significantly improved in 300-mg group compared with the pre-treatment (Figure 2). In 150-mg group, itopride significantly reduced the duration of longest episode compared with the pre-treatment. The individual values of acid reflux variable before and after treatment with 150 or 300 mg of itopride are given in Table 2.
| Variable | Group (mg/d) | Itopride Tx. | n | Mean | SD | P |
| Number of acid refluxes | 150 | Pre | 12 | 99.25 | 88.57 | 0.97 |
| Post | 12 | 76.92 | 60.53 | |||
| 300 | Pre | 13 | 150.77 | 246.15 | 0.057 | |
| Post | 13 | 52.23 | 39.79 | |||
| Number of acid refluxes longer than 5 min | 150 | Pre | 12 | 6.08 | 5.32 | 0.287 |
| Post | 12 | 3.83 | 5.32 | |||
| 300 | Pre | 13 | 6.23 | 5.12 | 0.054 | |
| Post | 13 | 2.69 | 2.5 | |||
| Longest time acid refluxes (min) | 150 | Pre | 12 | 30.55 | 33.37 | 0.018 |
| Post | 12 | 14.09 | 11.03 | |||
| 300 | Pre | 13 | 30.25 | 56.4 | 0.289 | |
| Post | 13 | 20.78 | 33.64 | |||
| Total time below pH 4 (min) | 150 | Pre | 12 | 138.42 | 98.56 | 0.16 |
| Post | 12 | 92.09 | 78.07 | |||
| 300 | Pre | 13 | 145.08 | 86.1 | 0.011 | |
| Post | 13 | 71.77 | 51.43 | |||
| Fraction time below pH 4 (%) | 150 | Pre | 12 | 11.13 | 9.26 | 0.211 |
| Post | 12 | 10.12 | 12.69 | |||
| 300 | Pre | 13 | 11.2 | 6.69 | 0.005 | |
| Post | 13 | 5.27 | 3.57 | |||
| DeMeester score | 150 | Pre | 12 | 32.5 | 16.96 | 0.16 |
| Post | 12 | 22.89 | 18.75 | |||
| 300 | Pre | 13 | 44.48 | 37.5 | 0.007 | |
| Post | 13 | 19.56 | 14.72 |
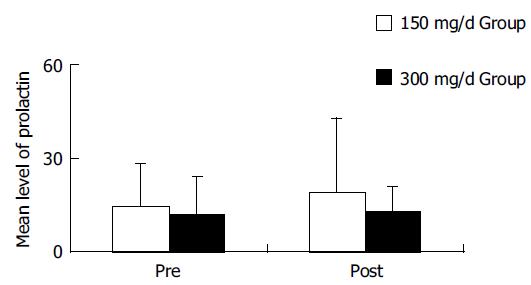
Itopride was well tolerated and only minor adverse events were reported. There were no significant changes in the mean level of prolactin with the 4-wk treatment of 150- or 300-mg itopride (Figure 3). In two patients of the 300-mg group, prolactin level was increased over 15 ng/mL, but there were no adverse events like lacteal secretion.
The result of this pilot study demonstrates that itopride decreases esophageal acid reflux and relieved symptoms without any significant adverse event in patients with GERD. These results show that itopride can improve the pathologic conditions of GERD.
The aims of treatment for GERD are relief of symptoms and healing of damaged lesion if endoscopic or pathologic examinations revealed mucosal damage of esophagus. Currently, it is being considered that long-term treatment is required because GERD has been regarded as chronic-relapsing disease like hypertension and diabetes mellitus[12,13].
Among several therapeutic drugs, a prokinetic agent has been used for the treatment and improvement of pathogenic mechanism of GERD such as gastrointestinal motility disorder, incompetent LES relaxation, impaired esophageal acid clearance, and prolonged gastric emptying. Among the kinds of prokinetic drugs, many studies have focused on the effect of cisapride on GERD. In one of them, cisapride increased tones of esophageal peristalsis and LES dose-dependency in healthy subjects[14,15]. On the contrary, in the other study, cisapride did not have any influence on the postprandial esophageal peristalsis, gastric acid[16] and LES pressure[17] in healthy subjects.
Even though this study does not confirm the efficacy of itopride on the esophageal peristalsis and LES, considering that itopride improves symptoms of the patients with GERD and decreases acid reflux effectively, we can speculate that itopride exerts some effects on improving esophageal motor dysfunction. Further studies will be required for making out the effect of itopride on the esophageal motor dysfunction of GERD.
From the viewpoint of improving symptom and healing mucosa, it was observed that administration of cisapride 10 mg four times a day (q.i.d.) and 20 mg twice a day (b.i. d.) improved the esophageal reflux compared with placebo, and cisapride had the same effect as H2 blocker on treatment of patients with mild esophagitis[6,7]. Additionally, it was reported that a combination therapy with cisapride and H2 blocker was more effective than monotherapy in patients with severe reflux esophagitis[18].
Although a few studies on functional dyspepsia show that the efficacy of itopride was equivalent to that of cisapride[11], this is the first study reporting that administration of itopride for 4 wk improves symptoms of GERD including heartburn (the main symptom of GERD) in both 150- and 300-mg group. So hereafter, additional studies will be required for evaluating the effect of itopride monotherapy or combination therapy with H2 receptor inhibitors on the esophagitis with mucosal damage as the initial and maintenance treatment.
The most standard method to measure the variation of esophageal acid is ambulatory 24-h pH-metry. In other studies using a similar method, cisapride 10 mg q.i.d. for 8 wk was more effective than placebo in terms of decreasing the number of reflux episodes, duration of longest episode, and percent time with pH < 4. These results suggest that cisapride is useful for acid reflux in patients with GERD[19].
In the study of mosapride 40 mg every day (q.d.) for 2 d, it reduced the number of reflux episodes, the number of reflux episodes > 5 min, and percent time with pH<4 and improved acid clearance time[20]. In this study, itopride 100 mg t.i.d. for 4 wk was effective in decreasing percent time with pH < 4 and DeMeester score. In this regard, it can be considered that itopride has a similar effect on decreasing acid reflux as cisapride or mosapride, although it is different in the action mechanism or duration of treatment from cisapride or mosapride[9,10].
The action mechanism of itopride on the reflux variables is unclear so far. But, dual mechanism of itopride, acetylcholine esterase inhibitory action and antagonistic activity on dopamine D2 receptor, which is known to inhibit activities of acetylcholine[21], seems to make this drug effective on promoting the esophageal and gastrointestinal movement and on improving acid clearance. It has been known that the threshold of itopride to promote gastrointestinal motility is higher than that of cisapride, metoclopramide and domperidone[8].
Prokinetic drugs that inhibit dopamine D2 receptor may elicit some adverse events such as fatigue, confusion, extrapyramidal symptom, and hyperprolactinemia that cause lacteal secretion or breast enlargement. Although there was no extensive study concerned about safety of itopride, the development of extrapyramidal symptom or hyperprolactinemia was uncommon with the administration of usual dosage of itopride (150 mg/d). In this study, there was no hyperprolactinemia and extrapyramidal symptoms in 150-mg group of itopride and there were two cases showing over 15 ng/mL of prolactin level in 300-mg group of itopride without adverse events like lacteal secretion[22].
Taken together, these results demonstrate that the administration of 150 or 300 mg of itopride for 4 wk improves symptoms of GERD and that the administration of 300 mg of itopride for 4 wk improves mild esophagitis and pathologic acid reflux. Therefore, itopride can be useful for treatment of the patients with GERD.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Lundell L, Myers JC, Jamieson GG. Is motility impaired in the entire upper gastrointestinal tract in patients with gastro-oesophageal reflux disease? Scand J Gastroenterol. 1996;31:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Rydberg L, Ruth M, Lundell L. Does oesophageal motor function improve with time after successful antireflux surgery? Results of a prospective, randomised clinical study. Gut. 1997;41:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Achem SR, Robinson M. A prokinetic approach to treatment of gastroesophageal reflux disease. Dig Dis. 1998;16:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Ramirez B, Richter JE. Review article: promotility drugs in the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1993;7:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Kim YB, Song CW, Kim HR, Lee SW, Bak YT, Hyun JH, Moon JS, Park HC. The incidence of gastroesophageal reflux disease and the effect of cisapride in patients with epigastric soreness. Korean J Gastrointestinal Motility. 2000;6:168-195. |
| 6. | Richter JE, Long JF. Cisapride for gastroesophageal reflux disease: a placebo-controlled, double-blind study. Am J Gastroenterol. 1995;90:423-430. [PubMed] |
| 7. | Castell DO, Sigmund C, Patterson D, Lambert R, Hasner D, Clyde C, Zeldis JB. Cisapride 20 mg b.i.d. provides symptomatic relief of heartburn and related symptoms of chronic mild to moderate gastroesophageal reflux disease. CIS-USA-52 Investigator Group. Am J Gastroenterol. 1998;93:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Iwanaga Y, Miyashita N, Morikawa K, Mizumoto A, Kondo Y, Itoh Z. A novel water-soluble dopamine-2 antagonist with anticholinesterase activity in gastrointestinal motor activity. Comparison with domperidone and neostigmine. Gastroenterology. 1990;99:401-408. [PubMed] |
| 9. | Iwanaga Y, Miyashita N, Mizutani F, Morikawa K, Kato H, Ito Y, Itoh Z. Stimulatory effect of N-[4-[2-(dimethylamino)-ethoxy] benzyl]-3,4-dimethoxybenzamide hydrochloride (HSR-803) on normal and delayed gastrointestinal propulsion. Jpn J Pharmacol. 1991;56:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Choi MG, Choo KY, Kim BW, Choi H, Park SH, Oh JH, Han SW, Kim JK, Chung IS, Chung KW. The effect of itopride on proximal gastric tone and visceral perception in healthy human. Kor J Gastroenterol. 2000;36:293-301. |
| 11. | Kim JK, Moon SB, Choi H, Kim SW, Chung KW, Sun HS, Lee HY, Hong SS. An effectiveness and safety of Itopride versus Cisapride in functional dyspepsia. Kor J Gastroenterol. 1999;33:749-756. |
| 12. | DeVault KR. Overview of medical therapy for gastroesophageal reflux disease. Gastroenterol Clin North Am. 1999;28:831-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 252] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Gilbert RJ, Dodds WJ, Kahrilas PJ, Hogan WJ, Lipman S. Effect of cisapride, a new prokinetic agent, on esophageal motor function. Dig Dis Sci. 1987;32:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ceccatelli P, Janssens J, Vantrappen G, Cucchiara S. Cisapride restores the decreased lower oesophageal sphincter pressure in reflux patients. Gut. 1988;29:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Wallin L, Kruse-Andersen S, Madsen T, Boesby S. Effect of cisapride on the gastro-oesophageal function in normal human subjects. Digestion. 1987;37:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Holloway RH, Downton J, Mitchell B, Dent J. Effect of cisapride on postprandial gastro-oesophageal reflux. Gut. 1989;30:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Galmiche JP, Brandstätter G, Evreux M, Hentschel E, Kerstan E, Kratochvil P, Reichel W, Schütze K, Soule JC, Vitaux J. Combined therapy with cisapride and cimetidine in severe reflux oesophagitis: a double blind controlled trial. Gut. 1988;29:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Nah YH, Song JH. Effect of Cisapride on ambulatory 24 - hour esophageal pH profile in gastroesophageal reflux disease. Kor J Gastroenterol. 1990;22:515-521. |
| 20. | Ruth M, Hamelin B, Röhss K, Lundell L. The effect of mosapride, a novel prokinetic, on acid reflux variables in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1998;12:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Glavin GB, Szabo S. Dopamine in gastrointestinal disease. Dig Dis Sci. 1990;35:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Perkel MS, Hersh T, Moore C, Davidson ED. Metoclopramide therapy in fifty-five patients with delayed gastric emptying. Am J Gastroenterol. 1980;74:231-236. [PubMed] |