Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4206
Revised: January 10, 2005
Accepted: January 13, 2005
Published online: July 21, 2005
AIM: Complete resection of the bile duct carcinoma is sometimes difficult by subepithelial spread in the duct wall or direct invasion of adjacent blood vessels. Nonresected extrahepatic bile duct carcinoma has a dismal prognosis, with a life expectancy of about 6 mo to 1 year. To improve the treatment results of locally advanced bile duct carcinoma, we have been conducting a clinical trial using regional hyperthermia in combination with chemoradiation therapy.
METHODS: Eight patients complaining of obstructive jaundice with advanced extrahepatic bile duct underwent thermo-chemo-radiotherapy (TCRT). All tumors were located in the upper bile duct and involved hepatic bifurcation, and obstructed the bile duct completely. Radiofrequency capacitive hyperthermia was administered simultaneously with chemotherapeutic agents once weekly immediately following radiotherapy at 2 Gy. We administered heat to the patient for 40 min after the tumor temperature had risen to 42°C. The chemothe-rapeutic agents employed were cis-platinum (CDDP, 50 mg/m2) in combination with 5-fluorouracil (5-FU, 800 mg/m2) or methotrexate (MTX, 30 mg/m2) in combination with 5-FU (800 mg/m2). Number of heat treatments ranged from 2 to 8 sessions. The bile duct at autopsy was histologically examined in three patients treated with TCRT.
RESULTS: In respect to resolution of the bile duct, there were three complete regression (CR), two partial regression (PR), and three no change (NC). Mean survival was 13.2 ± 10.8 mo (mean±SD). Four patients survived for more than 20 mo. Percutaneous transhepatic biliary drainage (PTBD) tube could be removed in placement of self-expandable metallic stent into the patency-restored bile duct after TCRT. No major side effects occurred. At autopsy, marked hyalinization or fibrosis with necrosis replaced extensively bile duct tumor and wall, in which suppressed cohesiveness of carcinoma cells and degenerative cells were sparsely observed.
CONCLUSION: Although the number of cases is rather small, TCRT in the treatment of locally advanced bile duct carcinoma is promising in raising local control and thus, long-term survival.
- Citation: Kamisawa T, Tu Y, Egawa N, Karasawa K, Matsuda T, Tsuruta K, Okamoto A. Thermo-chemo-radiotherapy for advanced bile duct carcinoma. World J Gastroenterol 2005; 11(27): 4206-4209
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4206.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4206
Extrahepatic bile duct carcinomas are typically slow-growing and locally invasive tumors, which spread along nerves and invade adjacent vascular structures[1]. Optimal treatment of these tumors includes complete surgical resection with negative histologic margins[2]. However, complete resection is sometimes difficult by local extension, which occur perineurally, via lymphatic channels, by subepithelial spread in the duct wall, and by direct invasion of adjacent blood vessels[3]. Nonresected extrahepatic bile duct carcinoma has a dismal prognosis, with a life expectancy of about 6 mo to 1 year[4].
Hyperthermia has been used in combination with radiation therapy and chemotherapy, and is considered to be effective for certain type of tumors[5]. We have been conducting a clinical trial of regional hyperthermia in combination with chemoradiotherapy for advanced extrahepatic bile duct carcinoma. In this study, clinical effectiveness of thermo-chemo-radiotherapy (TCRT) for locally advanced extrahepatic bile duct carcinoma was evaluated.
Eight patients complaining of obstructive jaundice with advanced extrahepatic bile duct underwent TCRT. All tumors were located in the upper bile duct and involved hepatic bifurcation. Bile duct was completely obstructed on percutaneous transhepatic or endoscopic retrograde cholangiography. Six patients underwent percutaneous transhepatic biliary drainage (PTBD). Unresectable reasons were involvement of the portal vein (n = 5) and extensive involvement of hepatic hilus (n = 3, Table 1). None of them showed distant metastasis.
| Case | Age (yr) | Sex | Unresectable reasons |
| 1 | 70 | m | Extensive involvement of hepatic hilus |
| 2 | 57 | m | Involvement of the portal vein |
| 3 | 73 | m | Extensive involvement of hepatic hilus |
| 4 | 70 | m | Involvement of the portal vein |
| 5 | 70 | f | Involvement of the portal vein |
| 6 | 78 | m | Involvement of the portal vein |
| 7 | 65 | m | Involvement of the portal vein |
| 8 | 71 | m | Extensive involvement of hepatic hilus |
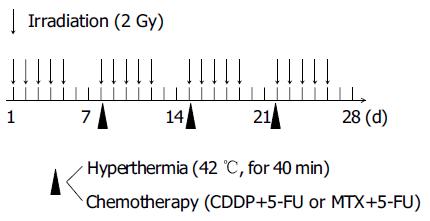
The heating equipment was radiofrequency (RF) capacitive heating device, Thermotron RF-8 (Yamamoto Vinita Company, Osaka, Japan)[6]. The patient lay in the prone position. The target was sandwiched with upper and lower electrodes, and 8 MHz RF wave was applied. We administered heat to the patient for 40 min after the tumor temperature had risen to 42°C. Tumor temperature was measured continuously using a needle thermosensor on each heating. The thermosensor was inserted beside the tumor from the skin surface through 18-G angiocatheter under the aid of ultrasonography. The chemotherapeutic agents employed were cis-platinum (CDDP, 50 mg/m2) in combination with 5-fluorouracil (5-FU, 800 mg/m2) or methotrexate (MTX, 30 mg/m2) in combination with 5-FU (800 mg/m2). The hyperthermia and chemotherapeutic agents were administered simultaneously once weekly immediately following radiotherapy at 2 Gy. Usually it started within 15 min after the irradiation (Figure 1). Number of heat treatments ranged from 2 to 8 sessions (mean±SD, 4.5 ± 2.5). Two cases were retreated (Table 2).
| Case | Heat session | Chemotherapy | Dose of radiation (Gy) |
| 1 | × 4, × 4 | MTX + 5Fu | 50 + 40 |
| 2 | × 2, × 6 | CDDP + 5Fu | 46 |
| 3 | × 6 | MTX + 5Fu | 56 |
| 4 | × 4 | MTX + 5Fu | 56 |
| 5 | × 4 | CDDP + 5Fu | 50 |
| 6 | × 2 | CDDP + 5Fu | 46 |
| 7 | × 2 | MTX + 5Fu | 50 |
| 8 | × 2 | MTX + 5Fu | 16 |
The effectiveness of TCRT on the advanced bile duct carcinoma was evaluated about resolution of biliary obstruction by cholangiography. Response of obstructed bile duct was graded as complete regression (CR: more than 80% resolution), partial regression (PR: more than 50% and less than 80% resolution), and no change (NC).
We examined histologically the state of the bile duct at autopsy in three patients with advanced bile duct carcinoma treated with TCRT.
Side effects were evaluated and graded according to the National Cancer Institute Common Toxicity Criteria[7].
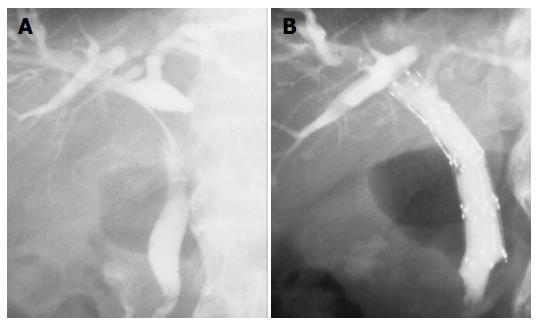
In respect to resolution of the bile duct, there were 3 CR, 2 PR, and 3 NC. CR rate and CR+PR rate was 38% and 63%. Mean survival was 13.2 ± 10.8 mo. Four patients survived for more than 20 mo (Table 3). We placed self-expandable metallic stent into the patency-restored bile duct for prevention of restenosis (n = 1) and partially resolved bile duct (n = 2) after TCRT for improvement of patients’ quality of life (Figures 2A and B). In these three patients, PTBD tube could be withdrawn.
| Case | Resolution of the bile duct | Survival months |
| 1 | Complete response | 33 |
| 2 | Complete response | 22 |
| 3 | Complete response | 22 |
| 4 | Partial response | 21.5 |
| 5 | Partial response | 6 |
| 6 | No change | 13.5 |
| 7 | No change | 4 |
| 8 | No change | 2.5 |
Marked hyalinization or fibrosis with necrosis replaced extensively bile duct tumor and wall, in which suppressed cohesiveness of carcinoma cells and degenerative cells were sparsely observed. Carcinoma cells were also detected peripherally. Common bile duct of two cases was not completely obstructed, though it was partly obstructed with debris or necrotic mass.
Treatment complications by TCRT were nausea and vomiting (grades 1 and 2, five cases), gastritis (grade 2, three cases), leukocytopenia (grade 2, three cases), and thrombocytopenia (grade 1, one case). These complications were successfully treated conservatively.
Optimal treatment of bile duct carcinoma is complete surgical resection with negative histologic margins[2]. However, complete resection is sometimes difficult by local extension, especially when the tumor located near the hepatic bifurcation. Deeply invaded tumors are apt to spread to the intrahepatic bile duct or the connective tissues in the hepatoduodenal ligament, with encasement of major vessels. Moreover, tumor cells that spread to the ligament often cannot be cleared away completely, even when dissection of the tissue is performed. In nonresected cases, tumor involving the hepatic hilus frequently induces the development of obstructive jaundice which cannot be under control, resulting in early death from cholangitis. One of the desirable strategies for advanced bile duct carcinoma appears to control over involvement of the hepatic hilar bile duct and the hepatoduodenal ligament.
We have treated advanced gallbladder carcinoma with TCRT and reported the effectiveness[8]. Then, we undertook a preliminary study involving TCRT for unresectable bile duct carcinomas. In respect to resolution of the bile duct, there were three CR and two PR of eight treated cases. Four patients survived for more than 20 mo. PTBD tube could be removed in three patients, in whom self-expandable metallic stent was placed into the patency-restored bile duct after TCRT.
Hyperthermia involves two biological interactions with radiation, a radiosensitizing effect[9,10] and a direct cytotoxic effect on tumor cells[11]. When the target lesion is heated up to around 42°C, the cancer-killing effect of radiation or anticancer drug is enhanced[12]. Experimental studies strongly suggest that the extracellular pH of the tumor and a hypoxic environment sensitize tumor cells to direct heat killing[13]. Hyperthermia in combination with radiotherapy for superficial malignancies has contributed to a better rate of complete response and reduction in the rate of recurrence compared with radiotherapy alone[14]. Data on hyperthermia for deep-seated tumors were still preliminary, because the heating and thermal measurement techniques were not yet established. However, the effectiveness of TCRT for deep-seated tumors has been recently revealed by prospective randomized studies[15,16]. The treatment protocol of TCRT was established and its effectiveness was evidenced by Sugimachi and his colleagues[17]. They treated esophageal carcinoma with TCRT and demonstrated significant improvement in the clinical effectiveness and 5-year survival ratio. Anatomically, the bile duct is located near the abdominal wall, which is advantageous to heating and thermal measurement. Thus, it seems that TCRT may be a favorable treatment strategy for advanced bile duct carcinoma.
In conclusion, although our clinical trial was preliminary and the number of patients was small, the results were better than we expected. We prefer HCRT in place of aggressive surgical approach for patients with locally advanced bile duct carcinoma.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Suzuki M, Takahashi T, Ouchi K, Matsuno S. The development and extension of hepatohilar bile duct carcinoma. A three-dimensional tumor mapping in the intrahepatic biliary tree visualized with the aid of a graphics computer system. Cancer. 1989;64:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Sakamoto E, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Uesaka K. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg. 1998;227:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 213] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | D'Angelica MI, Jarnagin WR, Blumgart LH. Resectable hilar cholangiocarcinoma: surgical treatment and long-term outcome. Surg Today. 2004;34:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Arcangeli G, Cividalli A, Nervi C, Creton G, Lovisolo G, Mauro F. Tumor control and therapeutic gain with different schedules of combined radiotherapy and local external hyperthermia in human cancer. Int J Radiat Oncol Biol Phys. 1983;9:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, Abe M. Radiofrequency capacitive hyperthermia for deep-seated tumors. II. Effects of thermoradiotherapy. Cancer. 1987;60:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Appendix A Grading of toxicity. Manual of oncologic therapeutics. Philadelphia: J.B. Lippincott 1989; . |
| 8. | Okamoto A, Tsuruta K, Ishiwata J, Isawa T, Kamisawa T, Tanaka Y. Treatment of T3 and T4 carcinomas of the gallbladder. Int Surg. 1996;81:130-135. [PubMed] |
| 9. | Arcangeli G, Casale C, Colistro F, Benassi M, Lovisolo G, Begnozzi L. One versus four heat treatments in combination with radiotherapy in metastatic mammary carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kim JH, Hahn EW, Ahmed SA. Combination hyperthermia and radiation therapy for malignant melanoma. Cancer. 1982;50:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Overgaard J. Effect of hyperthermia on malignant cells in vivo. A review and a hypothesis. Cancer. 1977;39:2637-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44:4721s-4730s. [PubMed] |
| 13. | Lin JC, Levitt SH, Song CW. Relationship between vascular thermotolerance and intratumor pH. Int J Radiat Oncol Biol Phys. 1992;22:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Perez CA, Gillespie B, Pajak T, Hornback NB, Emami B, Rubin P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: a Report by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1989;16:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Harima Y, Nagata K, Harima K, Oka A, Ostapenko VV, Shikata N, Ohnishi T, Tanaka Y. Bax and Bcl-2 protein expression following radiation therapy versus radiation plus thermoradiotherapy in stage IIIB cervical carcinoma. Cancer. 2000;88:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | van der Zee J, González González D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 577] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 17. | Sugimachi K, Matsuda H, Ohno S, Fukuda A, Matsuoka H, Mori M, Kuwano H. Long-term effects of hyperthermia combined with chemotherapy and irradiation for the treatment of patients with carcinoma of the esophagus. Surg Gynecol Obstet. 1988;167:319-323. [PubMed] |