Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4194
Revised: October 13, 2004
Accepted: October 18, 2004
Published online: July 21, 2005
AIM: The balance between oxidants and antioxidants can play an important role in the initiation and development of liver diseases. Recently, we have described a new automated method for the determination of total antioxidant capacity (TAC) in human serum and plasma.
METHODS: We measured TAC and corrected TAC (CTAC -abstraction of interactions due to endogenous uric acid, bilirubin and albumin) in 52 patients with chronic liver diseases (41 patients with primary biliary cirrhosis (PBC), 10 patients with chronic hepatitis C and 13 patients with viral HCV cirrhosis) as well as in 10 healthy controls. In 23 PBC patients measurement were also done 6 mo after treatment with ursodeoxycholic acid (UDCA). The TAC assay was based on a modification of the crocin bleaching assay. The results were correlated with routine laboratory measurements and the histological stage of PBC.
RESULTS: There were no significant differences in TAC between the various groups. However, CTAC was consi-derably increased in the PBC group compared to controls and cirrhotics. Analysis of these patients according to disease stages showed that this increase was an early phenomenon observed only in stages I and II compared to controls, cirrhotics and patients with chronic hepatitis C). After 6 mo of treatment with UDCA, levels of CTAC decreased to those similar to that of controls.
CONCLUSION: Patients in the early stages of PBC present with high levels of corrected total antioxidant capacity and this maybe related to the pathophysiology of the disease. UDCA treatment restores the levels of CTAC to control levels.
- Citation: Notas G, Miliaraki N, Kampa M, Dimoulios F, Matrella E, Hatzidakis A, Castanas E, Kouroumalis E. Patients with primary biliary cirrhosis have increased serum total antioxidant capacity measured with the crocin bleaching assay. World J Gastroenterol 2005; 11(27): 4194-4198
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4194.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4194
Evidence of enhanced production of free radicals or significant decrease of antioxidant defense have been reported in all types of liver damage. A large number of studies have focused on the pathogenetic significance of oxidative stress in liver injury as well as on therapeutic interventions with antioxidant and metabolic scavengers[1-4]. Primary biliary cirrhosis (PBC) is a chronic granulomatous cholestatic disorder characterized by a possible immunological attack on bile ducts leading to fibrosis, cirrhosis, liver failure, and death. Several studies suggest that oxidant stress plays a key role in the progression of this disease[5-7].
Circulation of oxidative molecules has been incriminated in lipoprotein oxidation and the generation of increased arterial deposits, ultimately leading to atherosclerosis[8-11]. However marked hypercholesterolemia, typical of severe longstanding cholestasis in PBC patients, is not associated with an excess risk of cardiovascular disease[12]. Since the antioxidant status in PBC has been reported to be compromised, with several important components of the antioxidant defense mechanism being significantly decreased[5] there is a disagreement between these data.
Recently, we have introduced a new automated method for the estimation of the plasma total antioxidant capacity[13]. In the present study we assayed and evaluated the levels of antioxidant capacity in patients with chronic liver diseases, by this method. In addition, we have also corrected these results per a number of analytes, directly affecting redox potential, thus introducing the concept of “corrected antioxidant capacity”. Our results indicate that, although the total antioxidant capacity of patients with chronic liver diseases do not differ significantly from normal subjects, the corrected antioxidant capacity is increased in PBC patients, indicating that a counterbalancing mechanism might be present.
The study included 41 Greek patients with PBC (35 women and 6 men, median age 62 years, range 31-85 years) followed up at the Department of Gastroenterology of the University Hospital of Heraklion, Crete. Thirty-seven of them were positive for antimitochondrial antibody (AMA)-M2 testing by immunofluorescence and by ELISA. All patients had typical biochemical pattern. Liver histology consistent with PBC was available in 38 patients. Three patients with clinical evidence of portal hypertension (esophageal varices) and positive AMA-M2 were not submitted to liver biopsy. At the time of diagnosis 25 patients (median age 58 years, range 31-71) were in histological stage I or II and 16 patients (median age 67 years, range 54-85) were in histological stage III or IV, according to Ludwig et al criteria[14]. Laboratory data and clinical details at the time of diagnosis and prior to initiation of UDCA therapy are shown in Table 1. All patients were negative for markers of hepatitis B and C.
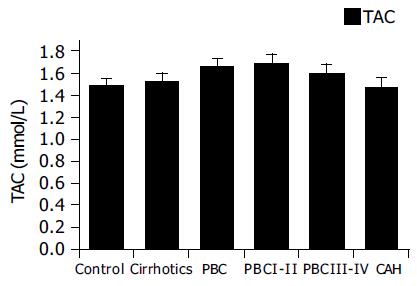
| Group | TAC (mmol/L) | CTAC (mmol/L) |
| Control | 1.493 ± 0.059 | 0.797 ± 0.057 |
| Cirrhotics | 1.532 ± 0.095 | 0.723 ± 0.069 |
| PBC | 1.664 ± 0.062 | 1.012 ± 0.068 |
| PBC I–II | 1.691 ± 0.080 | 1.096 ± 0.077 |
| PBC III–IV | 1.593 ± 0.086 | 0.789 ± 0.117 |
| CAH | 1.477 ± 0.080 | 0.875 ± 0.072 |
UDCA at a dose of 15 mg/(kg·d) was administered to all patients. Serum levels of TAC were measured in all patients prior to initiation of UDCA therapy. In 23 patients (14 in stages I-II and 9 in stages III-IV) TAC was also measured after 6 mo of UDCA therapy. No patients were withdrawn from treatment during this 6-mo period.
A group of 13 patients (8 men and 5 women, median age 53 years, range 41-64 years) with biopsy-proven postviral HCV liver cirrhosis was also enrolled. Another group of 10 patients (5 men and 5 women, median age 36 years, range 22-49 years), with biopsy-proven chronic hepatitis C was also included in the study as disease controls. No patient used alcohol. Finally, 10 healthy volunteers, matched to the PBC population for age and sex, served as a reference group.
In another group of 10 cirrhotic PBC patients and in all 13 control cirrhotics TAC levels estimations were also made in blood taken from the hepatic vein during catheterization for the measurement of wedged hepatic pressure done for assessment of portal hypertension. The group of PBC patients catheterized had been receiving UDCA treatment for at least 1 year.
Blood was collected from all patients and controls and was immediately centrifuged at 4°C. All serum samples were stored at -70°C until assayed. The study was approved by the local hospital Ethics committee, and written informed consent was obtained from all patients.
Plasma total antioxidant capacity (TAC) was measured on an Olympus AU-600 analyzer using the TAC kit (Medikon SA, Gerakas, Greece) as described previously[13]. Briefly, antioxidants in the sample inhibit the bleaching of crocin from ABAP [2,2-azobis-(2-amidinopropane) dihydrochloride] to a degree that is proportional to their concentration. The assay was performed at 37°C in the following steps: 2 µL of sample, calibrator or control were mixed with 250 µL of crocin reagent (R1) and incubated for 160 s. Subsequently, 250 µL of ABAP (R2) were added and the decrease in absorbance at 450 nm was measured 26 s later. Values of TAC were expressed as mmol/L.
Plasma uric acid, albumin, total bilirubin, alkaline phosphatase, aminotransferases, and γ-glutamyl transpeptidase were determined on an Olympus AU-600 analyzer using Olympus reagents provided by Medicon Hellas (Gerakas, Greece).
Statistical analysis of data was performed by the use of the SyStat v 10.0 program (SPSS Inc, Chicago, IL, USA), and the Origin v 5.0 program (MicroCal, Northampton, MA, USA). Paired and unpaired t-test were used where applicable. A P value less than 0.05 was considered statistically significant.
Figure 1 presents levels of TAC in the several groups tested. It appears that TAC values are slightly elevated in PBC patients, but this was not of statistical significance. No statistically significant differences between any of the groups could be identified. Since all patients did not have decompensated disease, our results show that the total antioxidant capacity is unaltered in non-hospitalized patients.
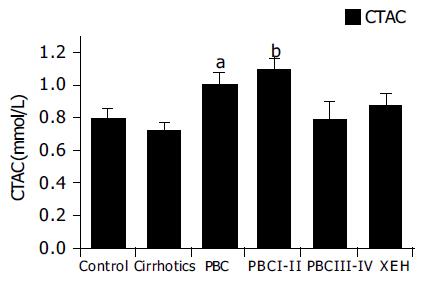
As TAC is a complex measurement, that is affected by a number of serum constituents, including bilirubin, we also calculated the corrected TAC. Corrected TAC (CTAC) is depicted in Figure 2. As stated in our previous work13, this calculated parameter represents the fraction of circulating antioxidants, after elimination of interference by endogenous metabolites. Our previous work has shown that uric acid and bilirubin, and in a lesser degree albumin, are the major endogenous substances linearly interfering with coefficients of 0.11, 0.11, and 0.01 mmol/L of TAC per mg/dL respectively. We have therefore assayed these analytes and subtracted their interferences from the obtained values of TAC to estimate possible modifications of the antioxidant activity, without those interferences. Increased levels of CTAC in patients with PBC compared to controls (P < 0.05) and cirrhotics (P < 0.01) were found (Figure 2). Further analysis shows that this increase is mainly attributed to early PBC patients (stages I and II P < 0.01, compared to controls, cirrhotics and patients with chronic hepatitis C), while late PBC patients (stages III and IV) had CTAC levels similar to controls.
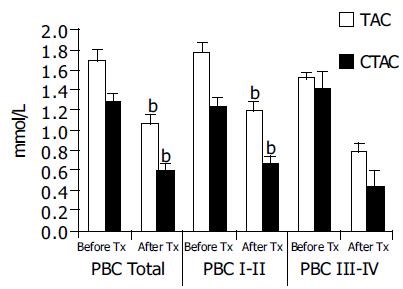
| Group | Before UDCA | After UDCA | Significance | |
| PBC Total | TAC | 1.697 ± 0.077 | 1.289 ± 0.093 | < 0.001 |
| CTAC | 1.068 ± 0.071 | 0.601 ± 0.093 | < 0.001 | |
| PBC I–II | TAC | 1.762 ± 0.082 | 1.243 ± 0.124 | < 0.001 |
| CTAC | 1.169 ± 0.076 | 0.660 ± 0.115 | < 0.001 | |
| PBC III–IV | TAC | 1.527 ± 0.184 | 1.410 ± 0.049 | NS |
| CTAC | 0.789 ± 0.153 | 0.448 ± 0.091 | NS |
In 23 patients that received 6 mo of UDCA therapy a reduction of both TAC and CTAC was noticed as shown in Figure 3. Reduction was significant in the early stages of PBC. In late stages, a reduction of CTAC was also noted but this was at the limit of statistical significance.
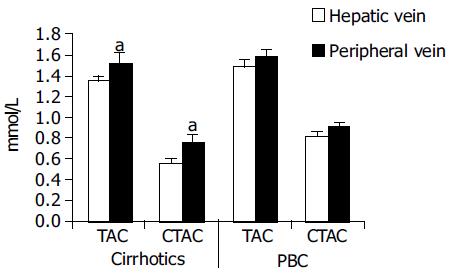
| Group | Hepatic | Peripheral | Significance | |
| Cirrhotics | TAC | 1.357 ± 0.052 | 1.532 ± 0.095 | < 0.05 |
| CTAC | 0.563 ± 0.031 | 0.757 ± 0.073 | < 0.05 | |
| PBC | TAC | 1.494 ± 0.074 | 1.587 ± 0.071 | NS |
| CTAC | 0.821 ± 0.032 | 0.913 ± 0.042 | NS |
Hepatic vein samples, taken during catheterization for estimation of portal hypertension had lower values of both TAC and CTAC as shown in Figure 4 compared to peripheral vein samples. This was significant only for the cirrhotic patients. In the treated PBC patients a similar trend was observed but it did not reach statistical significance.
Production of reactive species, including free radicals, is an integral part of human metabolism. Because of the high potential to damage vital biological systems, reactive species have now been incriminated in aging and in more than 100 disease states[15,16]. A complex system of neutralizing antioxidants exists in plasma and intra- and extracellular fluids, but an imbalance (oxidant stress) between free radical production and use can cause damage to DNA, lipids, proteins, and other biomolecules[5]. Nevertheless, most markers of oxidative damage have not been fully validated[17]. Endogenous antioxidant defenses (superoxide dismutases, H2O2-removing enzymes, metal binding proteins) are inadequate to prevent damage completely, so diet-derived antioxidants are important in maintaining health[16,18,19]. The sum of endogenous plus food-derived antioxidants represents the total antioxidant capacity of extracellular fluid. The overall antioxidant capacity may give more relevant biological information compared to that obtained by the measurement of individual parameters, as it considers the cumulative effect of all antioxidants present in plasma and body fluids[20].
A great variety of methods have been proposed for the assay of total antioxidant activity or capacity of serum or plasma[20,21]. Many researchers have stressed the distinction between antioxidant activity and antioxidant capacity: Antioxidant activity corresponds to the rate constant of a single antioxidant against a given free radical; antioxidant capacity, on the other hand, is the number of moles of a given free radical scavenged by a test solution, independently of the capacity of any one antioxidant present in the mixture. In the case of plasma being a heterogeneous solution of diverse antioxidants the antioxidant status is better reflected by antioxidant capacity rather than activity. This capacity is a combination of all redox chain antioxidants, including several analytes such as thiol bearing proteins, and uric acid. An increase of antioxidant capacity of plasma indicates absorption of antioxidants and improved in vivo antioxidant status[22], or the result of the activation of an adaptation mechanism to oxidative stress. It should be noted that, due to the participation of diverse metabolites to the antioxidant capacity of human plasma, its increase may not be necessarily a desirable condition. Indeed, in some cases, such as renal failure (uric acid), icteric status (bilirubin), hepatic damage (hypoalbuminemia) the variation of several metabolites modify plasma antioxidant capacity in a deleterious direction, a situation returning to normal values after correction of the underlying disease[23]. Recently, we have introduced a new automated method for the assay of the plasma antioxidant activity, based on crocin bleaching. This method (the TAC assay) gives an estimation of the integrated plasma antioxidant capacity. Furthermore, we also determine the interference of a number of endogenous analytes, such as uric acid, and bilirubin, which have been found to produce a major interference of TAC, while albumin results in a smaller interference[13].
In the present work, we have simultaneously assayed TAC and the concentrations of these analytes in patients with chronic liver diseases. As reported in the results no differences between TAC levels of the study groups were noted but patients in early stages of PBC had higher levels of CTAC. Other researchers have reported low total antioxidant capacity in PBC patients. Aboutwerat et al reported that oxidant stress, as reflected in a spectrum of lipid peroxidation and antioxidant markers, is a significant feature of early-stage PBC. They also reported that the total antioxidant capacity in whole serum (measured with an enhanced chemiluminescent technique) was significantly reduced in PBC patients but was normal in protein-free serum, suggesting that protein-bound components make an important contribution[5]. Floreani et al measured a number of antioxidant substances in both primary biliary cirrhosis and primary sclerosing cholangitis patients and reported significantly lower levels of retinol, alpha-tocopherol, total carotenoids, lutein, zeaxanthin, lycopene, alpha- and beta-carotene in these patients compared to healthy controls[24]. Discrepancies among these studies and our results could be attributed to different factors:
The different methods of assay of TAC, measuring a number of discrete molecules. The TAC assay, used in the present study is an integrator of the totality of antioxidants circulating at a given time point in the blood.
The distinction of activity and capacity discussed previously. Indeed, through chain oxidative-reduction reactions, a number of antioxidants measured at a given time point may not act as antioxidants per se[25].
Calculation of the corrected TAC (in which the interference of a number of endogenous metabolites has been subtracted) appears to provide a better estimate of the real antioxidant activity of the organism. However, the interpretation of the changes in plasma or serum antioxidant capacity depends upon not only the method used in detecting these changes, but also the conditions under which the plasma or serum antioxidant capacity is determined, because the determined antioxidant capacity reflects changes in a dynamic system. Furthermore an increased antioxidant capacity in plasma or serum is not necessarily a desirable condition if it is due to an adaptive response to increased oxidative stress at an early stage[21]. Our finding of increased CTAC in the early stages of PBC may reflect such an early response.
Ursodeoxycholic acid has been reported of having a marginal therapeutic effect for primary biliary cirrhosis[26]. Ursodeoxycholic acid treatment reduces intracellular hydrophobic bile acid levels and thereby may have a cytoprotective effect on cell membranes[27]. It has also been reported that UDCA reduced the deoxycholic acid-associated loss of the mitochondrial membrane potential and decreased the production of reactive oxygen species[28-30]. Our finding of TAC and CTAC reduction after UDCA treatment especially in the early stages of PBC maybe an indication that UDCA contributes to the normalization of the oxidative status in PBC patients.
Finally this is the first report that presents evidence of increased total antioxidant capacity originating from the periphery compared to the hepatic veins in patients with viral cirrhosis. Although the group of PBC patients used in this study had received UDCA treatment a similar trend can be noticed. Whether this is a feature of cirrhosis or a universal phenomenon cannot be concluded from our data. Although similar measurements cannot be performed in normal subjects, it would be interesting to further validate this result with studies on a bigger PBC population.
In conclusion, CTAC is considerably increased in the serum of PBC patients of stages I and II, but not in patients with viral cirrhosis or chronic hepatitis C. This increased antioxidant capacity in serum maybe due to an adaptive response to increased oxidative stress. Treatment with UDCA decreased the levels of CTAC to values similar to those of controls indicating a possible normalizing effect of UDCA on the oxidative status in PBC patients. Further study is needed for the finding of higher levels of CTAC in blood samples drawn from peripheral veins compared to samples from hepatic veins in cirrhotic patients.
G. Notas is a recipient of the Manasaki scholarship (University of Crete).
Science Editor Guo SY Language Editor Elsevier HK
Co-first-author: Niki Miliaraki
| 1. | Jones BE, Czaja MJ. III. Intracellular signaling in response to toxic liver injury. Am J Physiol. 1998;275:G874-G878. [PubMed] |
| 2. | Kaplowitz N. Mechanisms of liver cell injury. J Hepatol. 2000;32:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Mori N, Hirayama K. Long-term consumption of a methionine-supplemented diet increases iron and lipid peroxide levels in rat liver. J Nutr. 2000;130:2349-2355. [PubMed] |
| 5. | Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK, Warnes TW. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta. 2003;1637:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Ono M, Sekiya C, Ohhira M, Ohhira M, Namiki M, Endo Y, Suzuki K, Matsuda Y, Taniguchi N. Elevated level of serum Mn-superoxide dismutase in patients with primary biliary cirrhosis: possible involvement of free radicals in the pathogenesis in primary biliary cirrhosis. J Lab Clin Med. 1991;118:476-483. [PubMed] |
| 8. | Sigal E, Laughton CW, Mulkins MA. Oxidation, lipoxygenase, and atherogenesis. Ann N Y Acad Sci. 1994;714:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Stam H, Hülsmann WC, Jongkind JF, van der Kraaij AM, Koster JF. Endothelial lesions, dietary composition and lipid peroxidation. Eicosanoids. 1989;2:1-14. [PubMed] |
| 10. | Lyons TJ. Glycation and oxidation: a role in the pathogenesis of atherosclerosis. Am J Cardiol. 1993;71:26B-31B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Offermann MK, Medford RM. Antioxidants and atherosclerosis: a molecular perspective. Heart Dis Stroke. 1994;3:52-57. [PubMed] |
| 12. | Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, Podda M. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Kampa M, Nistikaki A, Tsaousis V, Maliaraki N, Notas G, Castanas E. A new automated method for the determination of the Total Antioxidant Capacity (TAC) of human plasma, based on the crocin bleaching assay. BMC Clin Pathol. 2002;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 528] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915-7922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3967] [Cited by in RCA: 3633] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 16. | Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598-620. [PubMed] |
| 17. | McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic Biol Med. 1999;26:1034-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 275] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 1686] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 19. | Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 991] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 20. | Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 677] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 21. | Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med. 1999;27:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 521] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 22. | Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998;68:1081-1087. [PubMed] |
| 23. | Jackson P, Loughrey CM, Lightbody JH, McNamee PT, Young IS. Effect of hemodialysis on total antioxidant capacity and serum antioxidants in patients with chronic renal failure. Clin Chem. 1995;41:1135-1138. [PubMed] |
| 24. | Floreani A, Baragiotta A, Martines D, Naccarato R, D'odorico A. Plasma antioxidant levels in chronic cholestatic liver diseases. Aliment Pharmacol Ther. 2000;14:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Barbaste M, Berké B, Dumas M, Soulet S, Delaunay JC, Castagnino C, Arnaudinaud V, Chèze C, Vercauteren J. Dietary antioxidants, peroxidation and cardiovascular risks. J Nutr Health Aging. 2002;6:209-223. [PubMed] |
| 26. | Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2002;1:CD000551. [PubMed] |
| 27. | Szalay F. Treatment of primary biliary cirrhosis. J Physiol Paris. 2001;95:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165-178. [PubMed] |
| 29. | Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 383] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 30. | Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ. Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ. 1999;6:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |